Functional consequences of developmentally regulated alternative splicing - PubMed (original) (raw)
Review
Functional consequences of developmentally regulated alternative splicing
Auinash Kalsotra et al. Nat Rev Genet. 2011.
Abstract
Genome-wide analyses of metazoan transcriptomes have revealed an unexpected level of mRNA diversity that is generated by alternative splicing. Recently, regulatory networks have been identified through which splicing promotes dynamic remodelling of the transcriptome to promote physiological changes, which involve robust and coordinated alternative splicing transitions. The regulation of splicing in yeast, worms, flies and vertebrates affects a variety of biological processes. The functional classes of genes that are regulated by alternative splicing include both those with widespread homeostatic activities and those with cell-type-specific functions. Alternative splicing can drive determinative physiological change or can have a permissive role by providing mRNA variability that is used by other regulatory mechanisms.
Figures
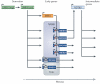
Figure 1. Role of splicing regulation during early meiosis in Saccharomyces cerevisiae
Starvation induces a switch in the Ume6p transcription factor from a repressor [UME6 (rep)] in vegetative cells to an activator [UME6 (act)] to initiate meiosis during sporulation. Ume6p activates multiple early meiotic genes including MER1 and 13 intron-containing genes. MER1 encodes an RNA binding protein that binds a motif near the 5′ splice site (orange box) and activates splicing of four intron-containing genes specifically during meiosis (orange arrow). Two Mer1p targets, MER3 and SPO22, are required for activation of NDT80, the transcriptional regulator of intermediate meiotic genes. Transcriptional regulation of MER1 and three of its four targets by UME6 delays expression of Mer1p-regulated genes compared to other UME6 targets creating a lag period prior to NDT80 activation. Mutation analyses demonstrated separate but overlapping meiotic-dependent splicing requiring MER1, NAM8 (blue shaded area), or TGS1 (grey shaded area).
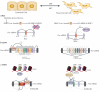
Figure 2. Integration of alternative splicing with epithelial-to-mesenchymal transitions
The scheme at the top represents epithelial and mesenchymal inter-conversion. Epithelial (left) and mesenchymal (right) splicing that directly influence EMT are depicted in the three panels below. (a) SRSF1 triggers EMT by promoting skipping of exon 11 of the Ron proto-oncogene to produce a constitutively active isoform (ΔRon) that confers an invasive phenotype. SRSF1 levels are dynamically controlled during EMT through AS-NMD by another splicing factor, Sam68. Epithelial cell-derived soluble factors repress ERK activity, thereby inhibiting Sam68 phosphorylation, which reduces SFRS1 levels through increased AS-NMD. (b) ESRP proteins are key regulators of the epithelial cell splicing network. ESRP1 downregulation leads to a switch from CD44(v)ariant to CD44(s)tandard isoforms that is crucial for EMT. Knockdown of all CD44 isoforms impaired EMT progression and the phenotype could be rescued by re-expressing the mesenchymal CD44s isoform, but not the epithelial CD44v isoform. CD44s and not CD44v potentiates Akt activation in TGFß-induced EMT assays, providing a functional link between alternative splicing and a key signaling pathway that drives EMT. (c) Mutually exclusive splicing of fibroblast growth factor receptor 2 (FGFR2) exons IIIb and IIIc is regulated by multiple splicing factors in communication with chromatin modifications. ESRP proteins inhibit exon IIIC while Rbfox2 promotes exon IIIb inclusion in epithelial cells-. PTB is expressed similarly in mesenchymal and epithelial cells but suppresses exon IIIb specifically in mesenchymal cells. The selective suppression is due to mesenchyme-specific enrichment of the H3K36me3 histone modification on chromatin near the IIIb exon. Binding of the adapter protein MRG15 to the H3K36me3 histone mark recruits PTB near exon IIIb resulting in IIIb skipping.
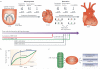
Figure 3. Coordinated alternative splicing changes drive fetal-to-adult transitions during postnatal heart development
(a) Two phases of mouse heart development. The morphogenesis phase occurs during early embryonic development; distinct cell populations from the first and second heart field migrate, divide, and proliferate to give rise to specific structures of the four-chambered heart. The morphogenesis phase is regulated by complex epigenetic, transcriptional and post-transcriptional networks including miRNAs. Growth and maturation in the second phase starts at E14.5 and is largely hypertrophic as cardiomyocytes exit the cell cycle and become post-mitotic. The importance of splicing networks during the second growth phase has become evident from mouse knockouts (MBNL1 and SRSF1, 2, 10)-, or transgenic expression (TgCELFΔ) of several splicing regulators in heart, which display late embryonic or a postnatal phenotypes, preceded by alternative splicing changes. The bottom shows timing of individual splicing regulator knockouts/transgenic expression indicated with colored lines and when splicing changes appear indicated by arrows. LV, left ventricle; RV, right ventricle; LA, left atrium; RA, right atrium; OFT, outflow tract; AA, aortic arch; PA, pulmonary artery. (b) Fetal-to-adult alternative splicing transitions are temporally coordinated to occur at specific times during development. (c) Members of CELF, MBNL, and SR family of splicing factors are regulators of splicing transitions that directly influence cardiac remodeling, EC coupling and cytoskeletal rearrangement. Postnatal up regulation of miRNAs suppress expression of multiple alternative splicing regulators which results in a physiological shift in fetal-to-adult splice patterns. In particular, postnatal up-regulation of two miRNAs that bind to the same seed sequence (miR-23a/b) causes the >10-fold down-regulation of CELF1 and CELF2 proteins.
Similar articles
- Diversification of stem cell molecular repertoire by alternative splicing.
Pritsker M, Doniger TT, Kramer LC, Westcot SE, Lemischka IR. Pritsker M, et al. Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14290-5. doi: 10.1073/pnas.0502132102. Epub 2005 Sep 23. Proc Natl Acad Sci U S A. 2005. PMID: 16183747 Free PMC article. - Rbm24 Regulates Alternative Splicing Switch in Embryonic Stem Cell Cardiac Lineage Differentiation.
Zhang T, Lin Y, Liu J, Zhang ZG, Fu W, Guo LY, Pan L, Kong X, Zhang MK, Lu YH, Huang ZR, Xie Q, Li WH, Xu XQ. Zhang T, et al. Stem Cells. 2016 Jul;34(7):1776-89. doi: 10.1002/stem.2366. Epub 2016 Mar 28. Stem Cells. 2016. PMID: 26990106 - Dynamic regulation of alternative splicing and chromatin structure in Drosophila gonads revealed by RNA-seq.
Gan Q, Chepelev I, Wei G, Tarayrah L, Cui K, Zhao K, Chen X. Gan Q, et al. Cell Res. 2010 Jul;20(7):763-83. doi: 10.1038/cr.2010.64. Epub 2010 May 4. Cell Res. 2010. PMID: 20440302 Free PMC article. - Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation.
Lopez AJ. Lopez AJ. Annu Rev Genet. 1998;32:279-305. doi: 10.1146/annurev.genet.32.1.279. Annu Rev Genet. 1998. PMID: 9928482 Review. - The functional consequences of intron retention: alternative splicing coupled to NMD as a regulator of gene expression.
Ge Y, Porse BT. Ge Y, et al. Bioessays. 2014 Mar;36(3):236-43. doi: 10.1002/bies.201300156. Epub 2013 Dec 18. Bioessays. 2014. PMID: 24352796 Review.
Cited by
- Repression of the Central Splicing Regulator RBFox2 Is Functionally Linked to Pressure Overload-Induced Heart Failure.
Wei C, Qiu J, Zhou Y, Xue Y, Hu J, Ouyang K, Banerjee I, Zhang C, Chen B, Li H, Chen J, Song LS, Fu XD. Wei C, et al. Cell Rep. 2015 Mar 10;10(9):1521-1533. doi: 10.1016/j.celrep.2015.02.013. Epub 2015 Mar 5. Cell Rep. 2015. PMID: 25753418 Free PMC article. - More than a pore: How voltage-gated calcium channels act on different levels of neuronal communication regulation.
Heck J, Palmeira Do Amaral AC, Weißbach S, El Khallouqi A, Bikbaev A, Heine M. Heck J, et al. Channels (Austin). 2021 Dec;15(1):322-338. doi: 10.1080/19336950.2021.1900024. Channels (Austin). 2021. PMID: 34107849 Free PMC article. Review. - Mutations in an AP2 transcription factor-like gene affect internode length and leaf shape in maize.
Jiang F, Guo M, Yang F, Duncan K, Jackson D, Rafalski A, Wang S, Li B. Jiang F, et al. PLoS One. 2012;7(5):e37040. doi: 10.1371/journal.pone.0037040. Epub 2012 May 23. PLoS One. 2012. PMID: 22649507 Free PMC article. - Circular RNAs are abundant, conserved, and associated with ALU repeats.
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Jeck WR, et al. RNA. 2013 Feb;19(2):141-57. doi: 10.1261/rna.035667.112. Epub 2012 Dec 18. RNA. 2013. PMID: 23249747 Free PMC article. - Quantification of stochastic noise of splicing and polyadenylation in Entamoeba histolytica.
Hon CC, Weber C, Sismeiro O, Proux C, Koutero M, Deloger M, Das S, Agrahari M, Dillies MA, Jagla B, Coppee JY, Bhattacharya A, Guillen N. Hon CC, et al. Nucleic Acids Res. 2013 Feb 1;41(3):1936-52. doi: 10.1093/nar/gks1271. Epub 2012 Dec 20. Nucleic Acids Res. 2013. PMID: 23258700 Free PMC article.
References
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. - PubMed
Publication types
MeSH terms
Grants and funding
- HL045565/HL/NHLBI NIH HHS/United States
- AR060733/AR/NIAMS NIH HHS/United States
- R01 AR045653/AR/NIAMS NIH HHS/United States
- R01 AR060733/AR/NIAMS NIH HHS/United States
- AR045653/AR/NIAMS NIH HHS/United States
- R01 HL045565/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases