Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation - PubMed (original) (raw)
Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation
Tadanori Mammoto et al. Dev Cell. 2011.
Abstract
Mesenchymal condensation is critical for organogenesis, yet little is known about how this process is controlled. Here we show that Fgf8 and Sema3f, produced by early dental epithelium, respectively, attract and repulse mesenchymal cells, which cause them to pack tightly together during mouse tooth development. Resulting mechanical compaction-induced changes in cell shape induce odontogenic transcription factors (Pax9, Msx1) and a chemical cue (BMP4), and mechanical compression of mesenchyme is sufficient to induce tooth-specific cell fate switching. The inductive effects of cell compaction are mediated by suppression of the mechanical signaling molecule RhoA, and its overexpression prevents odontogenic induction. Thus, the mesenchymal condensation that drives tooth formation is induced by antagonistic epithelial morphogens that manifest their pattern-generating actions mechanically via changes in mesenchymal cell shape and altered mechanotransduction.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
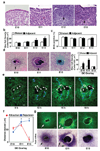
Figure 1. Induction of Pax9 expression and tooth formation by early dental epithelium (DE) in vitro and in vivo
a, Hematoxylin and eosin (H/E) stained histological sections showing development of the lower molar tooth in the mouse embryo. Tips of black arrows abut on the lower edge of the condensed mesenchyme. b, Graph shows mesenchymal cell densities measured in regions adjacent to (<83 µm) or distant (>83 µm) from the epithelial-mesenchymal interface (dashed lines). c, Graph showing the percent of BrdU-positive mesenchymal cells measured in regions adjacent to (<83 µm) or distant (>83 µm) from the epithelial-mesenchymal interface at each stage. d, H/E-stained cell cultures containing a retracted ball of intact DE isolated from E10, 11 or 13 embryos (dashed lines) overlaid atop a monolayer of cells isolated from the dental mesenchyme (DM) of E10 embryos (black arrows indicate regions of mesenchymal condensation). Graph at right shows mesenchymal cell densities measured in the regions adjacent to (<81 µm) or distant from (>81 µm) the epithelial-mesenchymal interface in vitro. e, Fluorescence micrographs showing images of GFP-labeled mesenchymal cells overlaid with E11 DE and cultured for 0, 12, 15 or 18 h. Note that the E11 DE (which is not visible in this view) repulsed underlying mesenchymal cells and caused them to move peripherally (migration paths of representative mesenchymal cells are indicated by white circles and dashed lines), while it simultaneously attracted other cells (indicated by blue circles and dashed lines) that moved closer to the epithelial-mesenchymal interface (light gray dashed lines) from distant regions of the mesenchymal cell monolayer (see Fig. S1b and Supplementary Movie M1). f, Graph shows results of quantifying total migration distances of mesenchymal cells that were attracted or repulsed in experiments using overlays containing DE isolated from E10, 11 or 13 embryos. g, Fluorescence microscopic (top) and in situ hybridization (ISH) (bottom) views showing the cell compaction and Pax9 mRNA localization, respectively, in the mesenchymal condensation induced when confluent GFP-mesenchymal cell monolayers are overlaid with DE isolated from E10, 11 or 13 embryos and cultured for 2 days in vitro (dashed lines indicate epithelial-mesenchymal interface) (see also Fig.S1f–h). In all figures, scale bars = 50 µm; *, p < 0.01.
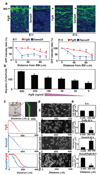
Figure 2. Fgf8 and Sema3f act as opposing short- and long-range morphogens that respectively stimulate and repulse mesenchymal cell migration
a, Fluorescence micrographs (top) showing the localization of Fgf8 (left) and Sema3f (right) proteins in mesenchyme at E11 (left) and E13 (right) (see also Fig.S2). Graphs (bottom) show the ratios of GFP intensities for Fgf8 (red circle) and Sema3f (blue square) measured in the mesenchyme at the indicated distances from BM, and in the DE adjacent to the BM (distance 0). b, The effects of different concentrations of Fgf8 (500, 250, 100, 50, 25 and 0 ng/ml) on mesenchymal cell migration for 16 h quantified using a Transwell migration assay. c, A microfluidic device fabricated in PDMS (left) containing microfluidic mixers that was used to create gradients of soluble molecules within a single flow channel, as visualized here by infusing FITC-dextran (Mr 70,000) (right). Graphs show the gradients of Fgf8 (top), Sema3f (middle) or Fgf8/Sema3f (bottom) that were applied to the cells in the device. Overlapping gradients of Fgf8 and Sema3f (bottom) were generated by adding an additional inlet channel to the left side of the last branching point of the original microfluidic gradient generator. d, The mesenchymal cells were plated in the microfluidic device and exposed to the gradients of Fgf8 and/or Sema3f with high concentrations at the left and low at the right, that was generated in the device by laminar flow. The upper phase contrast micrograph shows the localization of cells at time zero and 40 h after exposure to the gradients of Fgf8 (top), Sema3f (middle) and Fgf8/Sema3f (bottom); dashed circle indicates region of cell compaction. e, Graph showing the cell densities in the corresponding areas from e at 0 (top) or 40 h (bottom) in each treatment. Scale bars = 20 µm for a; 100 µm for c; 50 µm for d; *, p < 0.01.
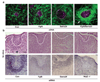
Figure 3. Early dental epithelium uses Fgf8 and Sema3f to induce mesenchymal condensation and Pax9 expression
a, Fluorescence (top) micrographs showing cultures containing labeled HEK 293 cell pellets that were being transfected with control (Con), Fgf8, Sema3f or both Fgf8 and Sema3f cDNAs, and overlaid on top of a GFP-labeled mesenchymal cell monolayer for 2 Ds (magenta, labeled HEK293 cell pellets; dashed line indicates pellet-mesenchymal cell interface). Note that only cells expressing both Fgf8 and Sema3f caused mesenchymal condensation around the pellet (white arrow) (see also Fig. S3b). b, Light micrographs of H/E-stained tissue sections (top) and Pax9 ISH images (bottom) showing tooth germs in E13 embryos treated with control (Con), Fgf8 or Sema3f siRNA in utero, or similar stage tooth germs from Nrp2 knockout (−/−) mice (see also Fig.S3c–e). Dashed lines indicate the epithelial-mesenchymal interface and tip of black arrows abut on the lower edge of the condensed mesenchyme. In all figures: scale bars=50 µm.
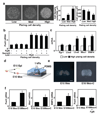
Figure 4. Mechanical control of odontogenic transcription factors during tooth development
a, Phase contrast micrographs (left) showing mesenchymal cells cultured for 16 h on microfabricated circular FN islands (500 µm diameter) in vitro at low, medium or high plating cell density (0.2, 1.2 or 2.4 × 105 cells/cm2, respectively), and graphs at right showing corresponding cell densities and projected cell areas measured under each condition. b, Graph showing Pax9 induction in mesenchymal cells cultured for 16 h on the circular FN islands (500 µm diameter) at low or high plating density with or without Fgf8 (150 ng/ml) and/or Sema3f (150 ng/ml). c, Graph showing induction of additional odontogenic factors (Egr1, Lhx6, Lhx8, Msx1 and BMP4) in mesenchymal cells cultured under the same conditions as in b. d, Freshly isolated E10 mesenchyme from first pharyngeal arch was physically compressed (1 kPa) for 16 h using a mechanical compressor composed of two pieces of PDMS polymer that are overlaid with a metal weight (see also Fig.S4f). e, Macroscopic images of the mesenchyme that was cultured ex vivo for 16 h in the absence (E10 Mes) or presence of compression (E10 Mes+C). f, Graph showing expression of Pax9, Msx1 and BMP4 mRNAs in control (E10 Mes) versus compressed mesenchyme (E10 Mes+C) and expression of Pax9 in control versus mesenchyme treated with soluble Fgf8 (150 ng/ml) for 16 h. In all figures: scale bars = 50 µm for a; 500 µm for e; *, p < 0.01.
Figure 5. Odontogenic induction is controlled by cell shape distortion and mediated by RhoA
a, Phase contrast micrographs showing spread cells cultured for 16 h on an unpatterned FN substrate (left) versus round mesenchymal cells cultured on small (20 µm diameter) circular FN islands (right). b, ISH views (left) and qRT-PCR (right) analysis showing Pax9 induction in mesenchymal cells cultured under the same conditions as in a. c, Graphs showing Pax9 induction in cells cultured for 16 h at low or high density (0.2 or 2.4 × 105 cells/cm2, respectively) on the circular FN island (500 µm diameter) in the absence or presence of SU5402 (left), and migration of the mesenchymal cells towards Fgf8 (150 ng/ml) measured using a Transwell migration assay with or without SU5402 (25 µM) (right). d, Graph showing Pax9 induction in Nrp2 −/− mesenchymal cells isolated at E10 from Nrp2 −/− embryos is preserved when the cells were cultured under the same conditions as in c. e, Fluorescence immunomicrographs showing the actin cytoskeleton in mesenchymal cells cultured under the same conditions as in c. Graph shows quantification of RhoA activity (ratio of active RhoA to total RhoA protein) in these mesenchymal cells (see also Fig.S5b). Cells were treated with virus encoding constitutively active RhoA (Rho CA virus) and analyzed as in b. Note that even though stress fibers do not form in the compact round cells (f), the Pax9 induction response was inhibited by constitutively active RhoA (g). h, Fluorescence micrographs (left) and ISH (right) views showing mesenchymal cell condensation (white arrows) and Pax9 induction (black arrow), respectively, in cultured GFP-labeled mesenchymal cells transduced with control (Con) virus or Rho CA virus and overlaid with E11 DE for 2 days. Note that increased RhoA activity suppressed Pax9 induction (see also Fig.S5d). In all figures: dashed lines indicate the epithelial-mesenchymal interface; scale bars = 20 µm for a, b, e, f and g; 50 µm for h; *, p < 0.01.
Figure 6. Mechanochemical control of tooth organ development
Fgf8 is produced by the dental epithelium (DE) and deposited in the basement membrane (BM) by E11 of development (left). The stored Fgf8, which is released over time (left to right), acts as a long-range morphogen to promote mesenchymal cell migration and to attract increased numbers of these cells toward the epithelial boundary. At the same time, the DE also produces local high concentrations of the repulsive morphogen Sema3f, which acts locally to repulse the migrating cells, causing them to crowd at the epithelial-mesenchymal interface (middle) and to form a well developed 'condensed mesenchyme' by E13 (right). The resulting compaction and physical compression of the mesenchymal cells (middle to right) is sufficient to induce expression of critical odontogenic genes, including Pax9, Msx1 and BMP4, which drive subsequent tooth organ formation. This mechanotransduction response is mediated by the cytoskeletal signaling molecule RhoA, which is suppressed in compressed cells within the condensed mesenchyme (not shown).
Comment in
- Squeezing into differentiation.
Ladher RK. Ladher RK. Dev Cell. 2011 Oct 18;21(4):607-8. doi: 10.1016/j.devcel.2011.09.017. Dev Cell. 2011. PMID: 22014519
Similar articles
- Functional consequences of interactions between Pax9 and Msx1 genes in normal and abnormal tooth development.
Ogawa T, Kapadia H, Feng JQ, Raghow R, Peters H, D'Souza RN. Ogawa T, et al. J Biol Chem. 2006 Jul 7;281(27):18363-9. doi: 10.1074/jbc.M601543200. Epub 2006 May 1. J Biol Chem. 2006. PMID: 16651263 - Mesenchymal condensation-dependent accumulation of collagen VI stabilizes organ-specific cell fates during embryonic tooth formation.
Mammoto T, Mammoto A, Jiang A, Jiang E, Hashmi B, Ingber DE. Mammoto T, et al. Dev Dyn. 2015 Jun;244(6):713-23. doi: 10.1002/dvdy.24264. Epub 2015 Apr 24. Dev Dyn. 2015. PMID: 25715693 Free PMC article. - Osr2 acts downstream of Pax9 and interacts with both Msx1 and Pax9 to pattern the tooth developmental field.
Zhou J, Gao Y, Zhang Z, Zhang Y, Maltby KM, Liu Z, Lan Y, Jiang R. Zhou J, et al. Dev Biol. 2011 May 15;353(2):344-53. doi: 10.1016/j.ydbio.2011.03.012. Epub 2011 Mar 17. Dev Biol. 2011. PMID: 21420399 Free PMC article. - Genes affecting tooth morphogenesis.
Kapadia H, Mues G, D'Souza R. Kapadia H, et al. Orthod Craniofac Res. 2007 Aug;10(3):105-13. doi: 10.1111/j.1601-6343.2007.00395.x. Orthod Craniofac Res. 2007. PMID: 17651126 Corrected and republished. Review. - Teeth. Where and how to make them.
Peters H, Balling R. Peters H, et al. Trends Genet. 1999 Feb;15(2):59-65. doi: 10.1016/s0168-9525(98)01662-x. Trends Genet. 1999. PMID: 10098408 Review.
Cited by
- The importance of foetal movement for co-ordinated cartilage and bone development in utero : clinical consequences and potential for therapy.
Shea CA, Rolfe RA, Murphy P. Shea CA, et al. Bone Joint Res. 2015 Jul;4(7):105-16. doi: 10.1302/2046-3758.47.2000387. Bone Joint Res. 2015. PMID: 26142413 Free PMC article. Review. - Self-organizing human cardiac microchambers mediated by geometric confinement.
Ma Z, Wang J, Loskill P, Huebsch N, Koo S, Svedlund FL, Marks NC, Hua EW, Grigoropoulos CP, Conklin BR, Healy KE. Ma Z, et al. Nat Commun. 2015 Jul 14;6:7413. doi: 10.1038/ncomms8413. Nat Commun. 2015. PMID: 26172574 Free PMC article. - Crosstalk between mechanotransduction and metabolism.
Romani P, Valcarcel-Jimenez L, Frezza C, Dupont S. Romani P, et al. Nat Rev Mol Cell Biol. 2021 Jan;22(1):22-38. doi: 10.1038/s41580-020-00306-w. Epub 2020 Nov 13. Nat Rev Mol Cell Biol. 2021. PMID: 33188273 Review. - Wnt5a and Notum Influence the Temporal Dynamics of Cartilaginous Mesenchymal Condensations in Developing Trachea.
Bottasso-Arias N, Mohanakrishnan M, Trovillion S, Burra K, Russell NX, Wu Y, Xu Y, Sinner D. Bottasso-Arias N, et al. bioRxiv [Preprint]. 2024 Nov 4:2024.09.02.610014. doi: 10.1101/2024.09.02.610014. bioRxiv. 2024. PMID: 39282283 Free PMC article. Preprint. - Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics.
Mammoto A, Mammoto T, Kanapathipillai M, Wing Yung C, Jiang E, Jiang A, Lofgren K, Gee EP, Ingber DE. Mammoto A, et al. Nat Commun. 2013;4:1759. doi: 10.1038/ncomms2774. Nat Commun. 2013. PMID: 23612300
References
- Angele P, Schumann D, Angele M, Kinner B, Englert C, Hente R, Fuchtmeier B, Nerlich M, Neumann C, Kujat R. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41:335–346. - PubMed
- Bei M, Kratochwil K, Maas RL. BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development. 2000;127:4711–4718. - PubMed
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. - PubMed
- Carter DR, Wong M. Mechanical stresses and endochondral ossification in the chondroepiphysis. J Orthop Res. 1988;6:148–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases