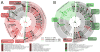The genome of th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment - PubMed (original) (raw)
The genome of th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment
Andrew Sczesnak et al. Cell Host Microbe. 2011.
Abstract
Perturbations of the composition of the symbiotic intestinal microbiota can have profound consequences for host metabolism and immunity. In mice, segmented filamentous bacteria (SFB) direct the accumulation of potentially proinflammatory Th17 cells in the intestinal lamina propria. We present the genome sequence of SFB isolated from monocolonized mice, which classifies SFB phylogenetically as a unique member of Clostridiales with a highly reduced genome. Annotation analysis demonstrates that SFB depend on their environment for amino acids and essential nutrients and may utilize host and dietary glycans for carbon, nitrogen, and energy. Comparative analyses reveal that SFB are functionally related to members of the genus Clostridium and several pathogenic or commensal "minimal" genera, including Finegoldia, Mycoplasma, Borrelia, and Phytoplasma. However, SFB are functionally distinct from all 1200 examined genomes, indicating a gene complement representing biology relatively unique to their role as a gut commensal closely tied to host metabolism and immunity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
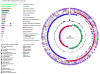
Figure 1. Circular representation of the SFB genome
Wheel
: The 5 contigs were arranged in order in a circular pseudochromosome (see Methods). Circles from outside in are: (i) ORF homology. Each ORF was color coded according to the genera most prevalent in its top ten PSI-BLAST hits (see left bottom corner for color legend). The SFB genome is dominated by ORFs homologous to Clostridium spp.; (ii) KEGG BRITE functional categories of annotated coding sequences (CDS) colored by type of category (listed on the left side of the figure); (iii) CDS on the forward strand; (iv) CDS on the reverse strand; (v) non-coding RNAs; (vi) markers delineating end of contigs; (vii) G-C content, and (viii) G-C skew, defined as (G − C) / (G + C).
Top Left:
KEGG BRITE categories in SFB and the 30 available Clostridium spp. genomes. Percentage of genes in each BRITE category. Top column in each category - percentage in the SFB genome, bottom column - average percentage in Clostridium spp. Arrows indicate the two categories that differ in SFB.
Figure 2. Genome-wide metabolic comparison between SFB and all sequenced genomes
Analysis of microbial functional similarities based on shared orthologous gene families (A,B) and modules (C). A/B. The 1,209 genomes in KEGG and the 13,118 KEGG Orthology gene families (KOs) are reported as circles and small cyan triangles, respectively. Organisms are connected by edges to all gene families contained within their genome. A) Global network of all genomes for visual overview. SFB (large red circle) lies outside any cluster but is close to groups of several Firmicutes genera and in particular Clostridium, Thermoanaerobacter, Staphylococcus, and Streptococcus. Other genera, including Mycoplasma, Borrelia, Treponema, Finegoldia and Gardnerella are quantitatively similar to SFB (see text) but located in the network periphery due to overall reduced gene content. Despite their similarity to SFB in terms of genome size and host environment, Helicobacter and Campylobacter are located in different regions of the network suggesting different functional specialization. B) Sub-network of SFB and the 20 most similar organisms (Tversky index 0.75, Table 2); KOs included in at least two organisms are depicted. SFB is functionally distinct from both the cluster of Clostridia (further differentiated as C. botulinum, C. perfringens, and “other”) and the Thermoanaerobacteria. C) Metabolic comparison based on functional genomic potential (Table 2) highlights SFB’s similarity to several Myco/Phyto/Acholeplasma and to Finegoldia magna (for which only one genome is available). The width and color (white = low, green = medium, red = high) of edges reflect relationship strength. Again, despite considering only the most functionally similar organisms, SFB is not directly included in the clusters they form.
Figure 3. KEGG modules over- and under-represented in the SFB genome compared to its 20 most similar organisms
Overview of the KEGG functional hierarchy; highlighted leaf nodes represent metabolic modules over- (green) or under-enriched (red) in SFB relative to the 20 most functionally similar organisms. Black circles represent modules present at a similar level in SFB and these organisms (absolute z-score <1) and white circles are absent in both. Similar organisms were computed using A) Tversky index α = 0.75 (representing larger related genomes) and B) Tversky index α = 0.25 (smaller, more minimal related organisms) and are listed in Table 2. A complete list of KEGG modules differentiating SFB from functionally similar organisms can be found in Table S4A and S4B.
Figure 4. Predicted SFB metabolic pathways
Overview of SFB metabolic pathways. SFB are highly auxotrophic and have a few complete essential pathways mostly for utilization of glycans and monosaccharides. They have complete glycolysis and pentose phosphate pathways, but lack the TCA cycle. Although fatty acid biosynthesis pathways are present, fatty acid metabolism pathways are absent. Absent as well are most pathways for co-factor and amino acid biosynthesis with the exception of the interrelated pathways for lysine, aspartate, glutamate, asparagine, and glutamine as noted in the figure. In contrast, multiple oligosaccharide and metal ion (in particular iron) transport and utilization mechanisms are present in SFB, including PTS, ABC, and other transporters, as well as extracellular peptidases and glycosyl hydrolases, which are shown interacting with extracellular glycans. SFB appear to be able to digest the glycoprotein components of the mucus layer and import sugars released in the process via ABC transporters and TCS. Once imported, several enzymes prepare these substrates for glycolysis. All these pathways provide SFB with the ability to acquire multiple metabolites from the surrounding environment and the host. Within the cell, essential pathways leading from import of polysaccharides through production of peptidoglycan, fatty acids, reduced ferredoxin, and acetate are shown.
Figure 5. Presence of genomes of intestinal microorganisms in the MetaHIT human metagenome database
The WGS Illumina reads of 124 individual fecal samples in the MetaHIT database (Qin et al., 2010) were aligned to the SFB genome and six other reference genomes (Clostridium perfringens ATCC13124, Enterococcus faecalis V583, Enterococcus faecium TX1330, Escherichia coli MG1655, Lactobacillus johnsonii NCC533, and Methanobrevibacter smithii ATCC35061). Reads with alignment identity of 95% or higher were used to calculate the relative abundance (the percentage of mapped reads in total reads) and genome coverage (percentage of genome bases aligned to reads) for each genome. Heatmaps representing the relative abundance (A) and coverage (B) in each human sample for each of the seven organisms are shown. All organisms were detected in multiple (albeit not all samples) with the exception of SFB and E. faecium. SFB was the only organism not detected in any sample at thresholds of 0.02% abundance and 0.5% coverage (see text).
Similar articles
- Induction of Th17 cells by segmented filamentous bacteria in the murine intestine.
Farkas AM, Panea C, Goto Y, Nakato G, Galan-Diez M, Narushima S, Honda K, Ivanov II. Farkas AM, et al. J Immunol Methods. 2015 Jun;421:104-111. doi: 10.1016/j.jim.2015.03.020. Epub 2015 Apr 7. J Immunol Methods. 2015. PMID: 25858227 Free PMC article. - Complete genome sequences of rat and mouse segmented filamentous bacteria, a potent inducer of th17 cell differentiation.
Prakash T, Oshima K, Morita H, Fukuda S, Imaoka A, Kumar N, Sharma VK, Kim SW, Takahashi M, Saitou N, Taylor TD, Ohno H, Umesaki Y, Hattori M. Prakash T, et al. Cell Host Microbe. 2011 Sep 15;10(3):273-84. doi: 10.1016/j.chom.2011.08.007. Cell Host Microbe. 2011. PMID: 21925114 - Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation.
Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Goto Y, et al. Immunity. 2014 Apr 17;40(4):594-607. doi: 10.1016/j.immuni.2014.03.005. Epub 2014 Mar 27. Immunity. 2014. PMID: 24684957 Free PMC article. - Segmented filamentous bacteria, Th17 inducers and helpers in a hostile world.
Schnupf P, Gaboriau-Routhiau V, Sansonetti PJ, Cerf-Bensussan N. Schnupf P, et al. Curr Opin Microbiol. 2017 Feb;35:100-109. doi: 10.1016/j.mib.2017.03.004. Epub 2017 Apr 25. Curr Opin Microbiol. 2017. PMID: 28453971 Review. - Segmented Filamentous Bacteria - Metabolism Meets Immunity.
Hedblom GA, Reiland HA, Sylte MJ, Johnson TJ, Baumler DJ. Hedblom GA, et al. Front Microbiol. 2018 Aug 24;9:1991. doi: 10.3389/fmicb.2018.01991. eCollection 2018. Front Microbiol. 2018. PMID: 30197636 Free PMC article. Review.
Cited by
- Intestinal epithelial c-Maf expression determines enterocyte differentiation and nutrient uptake in mice.
Cosovanu C, Resch P, Jordan S, Lehmann A, Ralser M, Farztdinov V, Spranger J, Mülleder M, Brachs S, Neumann C. Cosovanu C, et al. J Exp Med. 2022 Dec 5;219(12):e20220233. doi: 10.1084/jem.20220233. Epub 2022 Sep 19. J Exp Med. 2022. PMID: 36121416 Free PMC article. - Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment.
Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Morgan XC, et al. Genome Biol. 2012 Apr 16;13(9):R79. doi: 10.1186/gb-2012-13-9-r79. Genome Biol. 2012. PMID: 23013615 Free PMC article. - Segmented filamentous bacteria-induced immune responses: a balancing act between host protection and autoimmunity.
Flannigan KL, Denning TL. Flannigan KL, et al. Immunology. 2018 May 17;154(4):537-46. doi: 10.1111/imm.12950. Online ahead of print. Immunology. 2018. PMID: 29771448 Free PMC article. Review. - Gut immune maturation depends on colonization with a host-specific microbiota.
Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. Chung H, et al. Cell. 2012 Jun 22;149(7):1578-93. doi: 10.1016/j.cell.2012.04.037. Cell. 2012. PMID: 22726443 Free PMC article. - Induction of Th17 cells by segmented filamentous bacteria in the murine intestine.
Farkas AM, Panea C, Goto Y, Nakato G, Galan-Diez M, Narushima S, Honda K, Ivanov II. Farkas AM, et al. J Immunol Methods. 2015 Jun;421:104-111. doi: 10.1016/j.jim.2015.03.020. Epub 2015 Apr 7. J Immunol Methods. 2015. PMID: 25858227 Free PMC article.
References
- Atarashi K, Umesaki Y, Honda K. Microbiotal influence on T cell subset development. Seminars in immunology. 2011;23:146–153. - PubMed
- Ausiello CM, Cerquetti M, Fedele G, Spensieri F, Palazzo R, Nasso M, Frezza S, Mastrantonio P. Surface layer proteins from Clostridium difficile induce inflammatory and regulatory cytokines in human monocytes and dendritic cells. Microbes Infect. 2006;8:2640–2646. - PubMed
- Barketi-Klai A, Hoys S, Lambert-Bordes S, Collignon A, Kansau I. Role of fibronectin binding protein A in Clostridium difficile intestinal colonization. J Med Microbiol. 2011;60:1155–1161. - PubMed
Publication types
MeSH terms
Grants and funding
- U54HG004969/HG/NHGRI NIH HHS/United States
- R00 DK085329/DK/NIDDK NIH HHS/United States
- 4R00DK85329-02/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- 1R01HG005969/HG/NHGRI NIH HHS/United States
- R01 HG005969/HG/NHGRI NIH HHS/United States
- 5RC2AR058986/AR/NIAMS NIH HHS/United States
- U54 HG004969/HG/NHGRI NIH HHS/United States
- R00 DK085329-02/DK/NIDDK NIH HHS/United States
- RC2 AR058986/AR/NIAMS NIH HHS/United States
- U54 HG004973/HG/NHGRI NIH HHS/United States
- 1U54HG004973-01/HG/NHGRI NIH HHS/United States
- U54 HG003273/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases