Driving opposing behaviors with ensembles of piriform neurons - PubMed (original) (raw)
Driving opposing behaviors with ensembles of piriform neurons
Gloria B Choi et al. Cell. 2011.
Abstract
Anatomic and physiologic studies have suggested a model in which neurons of the piriform cortex receive convergent input from random collections of glomeruli. In this model, odor representations can only be afforded behavioral significance upon experience. We have devised an experimental strategy that permits us to ask whether the activation of an arbitrarily chosen subpopulation of neurons in piriform cortex can elicit different behavioral responses dependent upon learning. Activation of a small subpopulation of piriform neurons expressing channelrhodopsin at multiple loci in the piriform cortex, when paired with reward or shock, elicits either appetitive or aversive behavior. Moreover, we demonstrate that different subpopulations of piriform neurons expressing ChR2 can be discriminated and independently entrained to elicit distinct behaviors. These observations demonstrate that the piriform cortex is sufficient to elicit learned behavioral outputs in the absence of sensory input. These data imply that the piriform does not use spatial order to map odorant identity or behavioral output.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
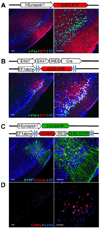
Figure 1. Expression of ChR2 in layer 2 and 3 of piriform cortex after injection with different variants of lentivirus encoding ChR2
A. Lentivirus carrying ChR2 fused to a fluorescent reporter (XFP = Cherry or EYFP) under control of the hSynapsin1 promoter was stereotactically injected into the piriform. The hSynapsin1 promoter drives ChR2:XFP expression in both excitatory and inhibitory neurons. Coronal sections through the injection site reveal expression of ChR2:XFP (red) in dense populations of layer 2 and 3 piriform neurons. The labeled cells are shown at higher magnification on the right. c-Fos expression after in vivo photostimulation is shown in green. NT = Neurotrace (blue). Scale bars on left = 50 µm and on right =100 µm. B. Lentivirus carrying ChR2:XFP flanked by loxP sites and under control of the EF1 alpha promoter was injected into the piriform of Emx1-IRES-Cre mice. ChR2:XFP (red) expression is restricted to dense populations of excitatory neurons in these mice. C. Lentivirus carrying ChR2:EYFP-IRES-nCherry (nuclear Cherry) flanked by loxP sites and under control of the EF1alpha promoter was co-injected into piriform with a second lentivirus carrying the hSynapsin1 promoter driving Cre:EGFP. This dual virus strategy was used to generate sparse labeling of piriform neurons. nCherry (red) labels the cell bodies whereas EYFP (green) labels both cell bodies and processes. D. c-Fos expression (blue) after in vivo photostimulation for the same animal shown in C.
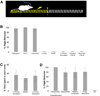
Figure 2. Ensembles of ChR2-expressing piriform neurons entrained to elicit aversive behavior
A. Schematic of the apparatus used for the aversive conditioning paradigm. During training, photostimulation of ChR2-expressing neurons in piriform, the conditioned stimulus (CS), was paired with foot shock, the unconditioned stimulus (US), applied only on the side of the arena where the animal was located at the time of photostimulation. The animals escaped foot shock by running to the opposite side. B. The percentage of trials in which animals exhibited flight behavior in response to the CS alone during the testing phase. hSynapsin1 = ChR2 expression driven from the human Synapsin1 promoter (N=7); Emx1 = ChR2 expression driven by the Emx1 promoter (N=4); Dual Virus = ChR2 expression generated by co-infection of Cre and Cre-dependent ChR2 viruses (animals with > 300 ChR2+ neurons, N=10); (−) Virus = no viral injection (N=3); EGFP = virus encoding EGFP but not ChR2 was injected into piriform (N=6); (−) Photostimulation = ChR2 expression driven by the hSynapsin1 promoter without photostimulation during training (N=3); Unpaired CS/US = ChR2 expression was driven from the human Synapsin1 promoter but foot shock application was not contingent upon photostimulation (i.e., equal numbers of CSs and USs were presented in random order with delays always exceeding 1 minute, N=4); Reversed CS/US = ChR2 expression was generated by the dual virus strategy but foot shock application preceded photostimulation. C. The percentage of time naïve ChR2-expressing animals spent in each chamber during a 5 minute (N=2) or 10 minute (N=8) testing period. One of the side chambers in a three-chambered arena was chosen as the (+) photostimulated compartment. Photostimulation was applied only when the animals entered the (+) photostimulated chamber. Training was not involved. ChR2 was densely expressed using the dual virus strategy. D. The percentage of trials in which ChR2-expressing animals (N=3) exhibited flight behavior in response to a complete multi-component CS or its components after training in the aversive conditioning paradigm. The complete CS was an odorant mix (ethyl acetate + citronellol) co-delivered with photostimulation. Odorant component was either ethyl acetate or citronellol. ChR2 was densely expressed using the dual virus strategy.
Figure 3. Efficiency of ChR2-expressing piriform neurons in eliciting conditioned behavior
A. Relationship between the number of ChR2-expressing neurons and incidence of flight behavior in the aversive conditioning paradigm. The number of ChR2+ neurons was determined and correlated with the percentage of trials in which animals exhibited flight behavior for mice expressing ChR2 using the dual virus strategy. B. Relationship between the number of ChR2-expressing neurons and distance traveled in the aversive conditioning paradigm. Same animals as in A. C. Spatial distribution of conditioned ensembles. The centers of ChR2-expressing ensembles are mapped on a schematic showing the borders of the piriform cortex for mice expressing ChR2 using the dual virus strategy and trained in the aversive conditioning behavioral paradigm. The borders of the piriform were drawn by referring to the Paxinos atlas. Only animals with > 300 ChR2+ neurons are included. Percent flight behavior for each injection site is documented in Table S1. D. Comparison of the number of CS-US pairings required for the onset of flight behavior in response to the CS alone in the aversive conditioning paradigm when the CS was either an odorant (N=4) or photostimulation of ChR2+ neurons (hSynapsin1: N=2, Emx1: N=2, Dual Virus with >300 ChR2+ neurons: N=7). E. Comparison of the number of blocks of trials required to reach a fraction of correct licks (# of licks following CS+ / total # of licks) exceeding 0.7 for two consecutive blocks in the appetitive go/no go discrimination assay when the CS was either an odorant (N=6) or photostimulation of ChR2+ neurons (Dual Virus with >300 ChR2+ neurons: N=6).
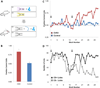
Figure 4. Ensembles of ChR2-expressing piriform neurons entrained to elicit appetitive behavior
A. Mice expressing ChR2 using the dual virus strategy were trained in an appetitive behavioral conditioning paradigm. Mice pre-trained to sample and lick only in response to a rewarded odor (CS+) subsequently received a water reward after photostimulation of ChR2-expressing neurons (CS+) but not in the absence of photostimulation (CS−). The CS+ and CS− were accompanied by a pulse of air to cue discrimination. Each training block consisted of 10 CS+ and 10 CS− trials. B. The average fraction of correct licks over the last three training blocks for ChR2-expressing animals (Emx1: N=1, Dual Virus with >300 ChR2+ neurons: N=6) and control animals (in which EGFP but not ChR2 was expressed or a Cre-dependent ChR2 virus was injected without a second Cre-expressing virus) trained using the same paradigm that included photostimulation (N=7). Fraction correct licks = # of licks following CS+ / total # of licks. C. Performance plotted as the fraction correct licks per block number for a ChR2-expressing mouse using the dual virus strategy and a control mouse in which EGFP but not ChR2 was expressed. Start of the training session on each day in C and D is marked with an arrow. D. Same data for the ChR2-expressing mouse shown in C plotted as the number of licks following the CS+ and CS−. The decrease in licks at the end of the first training session is typical and is likely due to the animal reaching satiety.
Figure 5. Ensembles of ChR2-expressing piriform neurons entrained to a socially rewarding stimulus
A. Entrainment of an odorant with a social reward. A male was trained in a three-chamber arena, in which the CS+ odor was paired with a female in a randomly selected side chamber (left). The other side chamber contained the CS− odor without a female. For testing, the animal was returned to the same arena with side chambers containing only the CS+ and CS− odors. On right, the percentage of time animals spent in each chamber during a 5 minute testing period is plotted for when CS+ and CS− were odors (N=6). CS+ = chamber with CS+ odor, Middle = middle chamber, CS− = chamber with CS- odor. B. Entrainment of ChR2-expressing ensembles with a social reward. During training, photostimulation was applied when the males actively investigated the female in a randomly selected side chamber (left). Upon testing in the absence of a female, photostimulation was delivered in one of the side chambers (CS+ chamber) but not the other (CS− chamber). On right, the percentage of time animals spent in each chamber during a 5 minute testing period when the CS+ was photostimulation is plotted for animals expressing ChR2 (ChR2, N=3) and for control animals without ChR2 (N=3). ChR2 was densely expressed using the dual virus strategy.
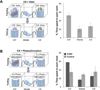
Figure 6. The same ensemble of ChR2-expressing neurons can be entrained to elicit appetitive and aversive behaviors
A. A schematic of the sequential training of ChR2-expressing animals to produce appetitive and aversive behaviors. B. A subset of mice shown in Figure 4B, which were trained in an appetitive water reward behavior, was subsequently trained in the aversive foot shock paradigm. The percentage of trials in which animals exhibited flight behavior in response to photostimulation alone during the testing phase is plotted for ChR2-expressing (Emx1: N=1, Dual Virus with >300 ChR2+ neurons: N=4) and control animals (N=6). C. The average lick number over the last three training blocks of sequentially trained animals (from 4B and 6B) before and after aversive conditioning with the same ensemble. ChR2 Before Aversive: number of licks following CS+ and CS− for ChR2-expressing mice during initial appetitive conditioning (CS+=58.68+9.76 licks and CS−=14.43+9.99 licks, N=5). ChR2 After Aversive: number of licks following CS+ and CS−for these mice after sequential appetitive-aversive conditioning (CS+=12.81+15.96 licks and CS−=11.39+8.21 licks, N=5). Control After Aversive: number of licks following CS+ and CS− for control animals after sequential appetitive-aversive conditioning (CS+=35.58±13.05 licks and CS−=34.29±4.94 licks, N=5).
Figure 7. Distinct ensembles of ChR2-expressing piriform neurons can be entrained to elicit different behaviors
A. A schematic of the apparatus used for the conditioning paradigm. Initially, stimulation of one ensemble (CS1) was paired with shock on the side of the arena where the animal received the photostimulation, and stimulation of the second ensemble (CS2) was paired with shock to the opposite side of the arena. For a reversal-learning paradigm, the shock contingency was switched between the two ensembles (Reversal Learning). B. The percentage of trials in which animals exhibited flight behavior in response to CS1 and CS2 after training with the CS-shock contingencies described in A, left (hSynapsin1: N=1, Dual Virus: N=4). C. The percentage of trials in which flight behavior was elicited by the CS1 and CS2 for a subset of animals shown in B after they were subsequently trained with reversed CS-shock contingencies described in A, right (Reversal Learning, hSynapsin1: N=1, Dual Virus: N=2).
Similar articles
- Transient and Persistent Representations of Odor Value in Prefrontal Cortex.
Wang PY, Boboila C, Chin M, Higashi-Howard A, Shamash P, Wu Z, Stein NP, Abbott LF, Axel R. Wang PY, et al. Neuron. 2020 Oct 14;108(1):209-224.e6. doi: 10.1016/j.neuron.2020.07.033. Epub 2020 Aug 21. Neuron. 2020. PMID: 32827456 Free PMC article. - Task-Demand-Dependent Neural Representation of Odor Information in the Olfactory Bulb and Posterior Piriform Cortex.
Wang D, Liu P, Mao X, Zhou Z, Cao T, Xu J, Sun C, Li A. Wang D, et al. J Neurosci. 2019 Dec 11;39(50):10002-10018. doi: 10.1523/JNEUROSCI.1234-19.2019. Epub 2019 Oct 31. J Neurosci. 2019. PMID: 31672791 Free PMC article. - Olfactory coding: random scents make sense.
Kay LM. Kay LM. Curr Biol. 2011 Nov 22;21(22):R928-9. doi: 10.1016/j.cub.2011.10.008. Curr Biol. 2011. PMID: 22115463 - Odor coding in piriform cortex: mechanistic insights into distributed coding.
Blazing RM, Franks KM. Blazing RM, et al. Curr Opin Neurobiol. 2020 Oct;64:96-102. doi: 10.1016/j.conb.2020.03.001. Epub 2020 May 15. Curr Opin Neurobiol. 2020. PMID: 32422571 Free PMC article. Review. - Odor representations in mammalian cortical circuits.
Isaacson JS. Isaacson JS. Curr Opin Neurobiol. 2010 Jun;20(3):328-31. doi: 10.1016/j.conb.2010.02.004. Epub 2010 Mar 5. Curr Opin Neurobiol. 2010. PMID: 20207132 Free PMC article. Review.
Cited by
- Perceiving invisible light through a somatosensory cortical prosthesis.
Thomson EE, Carra R, Nicolelis MA. Thomson EE, et al. Nat Commun. 2013;4:1482. doi: 10.1038/ncomms2497. Nat Commun. 2013. PMID: 23403583 Free PMC article. - An epigenetic trap stabilizes singular olfactory receptor expression.
Lyons DB, Allen WE, Goh T, Tsai L, Barnea G, Lomvardas S. Lyons DB, et al. Cell. 2013 Jul 18;154(2):325-36. doi: 10.1016/j.cell.2013.06.039. Cell. 2013. PMID: 23870122 Free PMC article. - Lavandula angustifolia Essential Oil and Linalool Counteract Social Aversion Induced by Social Defeat.
Caputo L, Reguilon MD, Mińarro J, De Feo V, Rodriguez-Arias M. Caputo L, et al. Molecules. 2018 Oct 19;23(10):2694. doi: 10.3390/molecules23102694. Molecules. 2018. PMID: 30347669 Free PMC article. - Diverse roles for the Drosophila fructose sensor Gr43a.
Miyamoto T, Amrein H. Miyamoto T, et al. Fly (Austin). 2014;8(1):19-25. doi: 10.4161/fly.27241. Epub 2013 Nov 22. Fly (Austin). 2014. PMID: 24406333 Free PMC article. - Bidirectional switch of the valence associated with a hippocampal contextual memory engram.
Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Redondo RL, et al. Nature. 2014 Sep 18;513(7518):426-30. doi: 10.1038/nature13725. Epub 2014 Aug 27. Nature. 2014. PMID: 25162525 Free PMC article.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. - PubMed
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. - PubMed
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 1999;24:637–645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases