Assembly of Saccharomyces cerevisiae 60S ribosomal subunits: role of factors required for 27S pre-rRNA processing - PubMed (original) (raw)
Assembly of Saccharomyces cerevisiae 60S ribosomal subunits: role of factors required for 27S pre-rRNA processing
Aarti Sahasranaman et al. EMBO J. 2011.
Abstract
The precise functions of most of the ∼200 assembly factors and 79 ribosomal proteins required to construct yeast ribosomes in vivo remain largely unexplored. To better understand the roles of these proteins and the mechanisms driving ribosome biogenesis, we examined in detail one step in 60S ribosomal subunit assembly-processing of 27SA(3) pre-rRNA. Six of seven assembly factors required for this step (A(3) factors) are mutually interdependent for association with preribosomes. These A(3) factors are required to recruit Rrp17, one of three exonucleases required for this processing step. In the absence of A(3) factors, four ribosomal proteins adjacent to each other, rpL17, rpL26, rpL35, and rpL37, fail to assemble, and preribosomes are turned over by Rat1. We conclude that formation of a neighbourhood in preribosomes containing the A(3) factors establishes and maintains stability of functional preribosomes containing 27S pre-rRNAs. In the absence of these assembly factors, at least one exonuclease can switch from processing to turnover of pre-rRNA.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
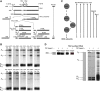
Pre-rRNA processing pathway in S cerevisiae and assembly factors involved in processing of 27SA3 pre-rRNA. (A) Pre-rRNA processing pathway in S. cerevisiae. The initial 35S pre-rRNA transcript, synthesized by RNA Polymerase I, contains sequences for mature 18S, 5.8S, and 25S rRNAs along with internal and external transcribed spacer sequences (ITS/ETS). Processing intermediates that form during ribosome assembly are indicated. Exo- and endonucleases, where known, are mentioned alongside the step in which they function. (B) Defects in pre-rRNA processing in A3 factor mutants. RNA was extracted from lysates prepared from cells grown in galactose (left lane in each pair) or shifted to glucose to deplete respective proteins (right lanes), and subjected to primer extension to assay the A2, A3, B1S, and B1L ends of pre-rRNAs. The B1S and B1L ends include both 27S and 7S pre-rRNA species. Depletion of Tif6 and Ebp2 are shown as controls for assembly mutants that do not specifically block processing of 27SA3 pre-rRNA. A wild-type control is included to indicate effects of the carbon source shift. Ratios of each processing intermediate in unshifted and shifted samples were quantified and are shown in Supplementary Table S3. All samples were run on the same gel, except GAL–ERB1, which was run on a separate gel with GAL–RLP7 as a control. (C) Timing of association of A3 factors with nascent ribosomes. The assembly pathway of 60S ribosomal subunits is shown. Preribosomes (grey circles) are aligned with processing intermediates in (A). Each line represents the duration of time for which an individual protein associates with preribosomes. The broken line indicates that the association of the protein with those preribosomal intermediates has not been determined. Arrowheads represent direction of the assembly pathway, and where determined, the point of exit of assembly factors. (D) Entry point of association of Rlp7, Nsa3/Cic1, and Ytm1 with nascent ribosomes. TAP-tagged strains as indicated were used to purify preribosomes containing Rlp7, Nsa3, or Ytm1. Pre-rRNAs present in these preribosomes were assayed by primer extension. Untagged parent strain is used as the negative control. ENP1–TAP strain is shown as positive control for co-IP of 35S pre-rRNA. Primer extension products for Rlp7 and Nsa3 samples were run on one gel, for Ytm1 on a second, and for Enp1 on a third, each with an untagged negative control. Figure source data can be found with the Supplementary Information.
Figure 2
A3 assembly factors are mutually interdependent for association with preribosomes. (A) Depletion of each of the six A3 factors results in mutant preribosomes with similar changes in protein constituents. Precursors to 60S subunits were isolated using TAP-tagged assembly factor Rpf2, from strains in which A3 factors were depleted using the conditional GAL promoter. Proteins present in the purified preribosomes were resolved by SDS–PAGE, and stained with silver. * Indicates proteins whose levels increase upon depletion of each protein, • indicates proteins whose levels decrease upon depletion of each protein. A3 factors are labelled. The GAL–REA1 RPF2–TAP strain is shown as a control for specificity of the phenotype. (B) Five other A3 factors are specifically missing from preribosomes in the absence of Rlp7. Western blotting was used to specifically assay the presence of selected proteins, including A3 factors, in mutant and wild-type preribosomes. (C) The Nop7 subcomplex remains intact, but is not associated with preribosomes in the absence of Rlp7. TAP-tagged Nop7 was used for affinity purification from wild-type (left lane) and Rlp7-depleted (right) extracts. Silver-stained protein bands corresponding to Nop7 subcomplex proteins Erb1, Nop7 and Ytm1 are indicated. (D) In the absence of Rrp1, the association of A3 factors with preribosomes is not measurably reduced. Proteins present in Rpf2–TAP-containing preribosomes purified from the GAL–RRP1 strain were assayed by SDS–PAGE, silver staining, and western blotting. rpL11 is the loading control.
Figure 3
iTRAQ analysis of changes in composition of preribosomes upon depletion of Rlp7. (A) Effects of depleting Rlp7 on ribosome assembly factors. The relative abundance of ribosome assembly factors is shown as the ratio from Rlp7-depleted cells to Rlp7-expressed cells (average relative ratio depletion/wild type). Light bars: proteins involved in 40S subunit assembly. Dark bars: proteins involved in 60S subunit biogenesis. s.e.m. are given. Results here and in (B) are from two independent biological replicates (two mutant, two wild type). The data were collected as a four-plex after a single LCLC MALDITOFTOF run. (B) Effects of depleting Rlp7 on ribosomal proteins. The relative abundance of r-proteins is shown as the ratio from Rlp7-depleted cells to Rlp7-expressed cells (average relative ratio depletion/wild type). Light bars: 40S ribosomal proteins. Dark bars: 60S ribosomal proteins. s.e.m. are given.
Figure 4
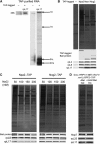
Recruitment of exonucleases Rat1, Xrn1, and Rrp17 to preribosomes. (A) Rrp17, but not Rat1 and Xrn1, is dependent on A3 factors to associate with preribosomes. The presence of Rat1, Xrn1, and Rrp17 in preribosomes purified from A3 factor mutants was assayed by SDS–PAGE and western blotting. rpL5 is the loading control. Binding of Rat1 to beads was not detected when extracts from untagged strains were used for purification (unpublished observations). (B) In the absence of Rai1, association of Rat1 with preribosomes is not affected. Preribosomes were purified from RAI1 (left) and _rai1_Δ (right) strains. Proteins were assayed by SDS–PAGE, silver staining, and western blotting. rpL5 is the loading control. (C) Generation of the 5′-end of 27SA3 pre-rRNA is not required for association of Rat1 with preribosomes. 66S preribosomes were isolated using TAP-tagged assembly factor Rpf2, from a strain in which RNase MRP was inactivated by depleting Pop3 using the conditional GAL promoter. Preribosomes were assayed by SDS–PAGE, silver staining, and western blotting against indicated proteins. (D) Rat1 is recruited to preribosomes early in the pathway of 60S subunit biogenesis. TAP-tagged Rat1 was used to purify Rat1-containing preribosomes. Pre-rRNAs present in these preribosomes were assayed by primer extension. In all, 50% of TAP-purified RNA was used to assay pre-rRNAs containing A2, A3, B1S, and B1L ends. The remaining RNA was used to detect 35S pre-rRNA (not shown).
Figure 5
Ribosomal proteins adjacent to 58S rRNA in mature ribosomes are missing from preribosomes lacking A3 factors. (A) Specific ribosomal proteins are reduced in preribosomes in the absence of Rlp7. Western blotting of ribosomal proteins in preribosomes purified from GAL–HA–RLP7 RPF2–TAP strain are shown. Indicated ribosomal proteins are 3xHA-tagged. R-proteins in the bottom panel were unaffected in the absence of Rlp7. (B) R-proteins dependent on A3 factors cluster around 5.8S rRNA. In both B and C, a 180° rotation of the crown view of the mature 60S subunit is shown. Domain I of rRNA is highlighted, with the region corresponding to 5.8S rRNA in blue and 25S rRNA in brown. Colour coding of ribosomal proteins: rpL17—purple, rpL26—teal, rpL35—green, rpL37—red. 5S rRNA is highlighted in orange. Pymol images here and in (C) were generated using PDB file 3O58 (Ben-Shem et al, 2010). Affected r-proteins are close to helices formed by basepairing between 5.8S and 25S rRNA. Only domain I of 5.8S/25S rRNAs is shown. rRNA and r-proteins are colour coded as in (B).
Figure 6
rpL17 becomes more stably associated with 66S preribosomes after 27SA3 pre-rRNA processing. (A) rpL17 is present in 27SA2 and 27SB containing preribosomes. rpL17–TAP strain was used to purify preribosomes containing rpL17. The untagged parent strain is used as a negative control. Pre-rRNAs that copurified were assayed by primer extension. The two samples on the left are from one gel and the two on the right are from a second gel. (B) rpL17 copurifies in larger amounts with ‘later’ 66S preribosomes than with ‘early’ 66S intermediates. Preribosomes were purified using indicated TAP-tagged early (Npa2) or middle assembly factors (Nsa1, Nog2), and assayed by SDS–PAGE, silver staining, and western blotting. Purification with the untagged parent strain is shown as a negative control. Purified bait proteins are indicated with asterisks. The higher molecular weight band in the untagged control strain corresponds to IgG heavy chain, and the lower molecular weight band is TEV protease. All protein samples were assayed on the same gel and blot. (C) Association of rpL17 with Npa2-containing preribosomes, but not Nog2-containing preribosomes, is salt sensitive. Preribosomes were purified using indicated TAP-tagged strains. After binding of cell lysates, beads were washed with buffer containing increasing concentrations of NaCl, as indicated. SDS–PAGE, silver staining, and western blotting were used to assay preribosomes. (D) Binding of rpL17 to preribosomes does not depend on processing of 27SA3 pre-rRNA. The three exonucleases required for 27SA3 pre-rRNA processing were depleted using the _GAL–RRP17 MET–RAT1 xrn1_Δ strain. The strain was grown in indicated media and preribosomes were purified using TAP-tagged Rpf2. Preribosomes were assayed by SDS–PAGE, silver staining, and western blotting. Figure source data can be found with the Supplementary Information.
Figure 7
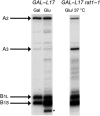
rpL17 is required for processing within ITS2, and to a smaller extent within ITS1. RNA was extracted from indicated strains grown in galactose or grown in galactose and shifted to glucose. The GAL–RPL17 rat1-1 strain was grown in glucose for 12 h at 25 °C to deplete rpL17 and then shifted to 37 °C for 5 h to inactivate Rat1-1. Primer extension was used to assay the A2, A3, B1S, and B1L ends of pre-rRNA. * Indicates 5′-truncated pre-rRNAs formed in the absence of rpL17. All samples were assayed on the same gel. Figure source data can be found with the Supplementary Information.
Figure 8
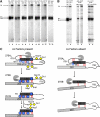
In the absence of A3 factors, Rat1 initiates turnover of misassembled preribosomes. (A) 5′-Truncated 27SB pre-rRNAs are formed in the absence of A3 factors. RNA was extracted from whole-cell lysates prepared from the indicated strains, and assayed by primer extension to detect A2, A3, B1S, and B1L 5′-ends. For each panel, asterisks indicate primer extension products that are 67–69 nts shorter than 27SB1S pre-rRNA at the 5′-end. All samples were run on the same gel, except GAL–ERB1, which was run won a separate gel with GAL–RLP7 as a control. (B) Rat1 activity is required for formation of the shorter pre-rRNAs. RNA was extracted and assayed as described in (A) above. The GAL–RPL17 rat1-1 strain was grown in glucose for 12 h at 25 °C to deplete rpL17 and then shifted to 37 °C for 5 h to inactivate Rat1-1. Samples from lanes 3 and 4 were run on the same gel. (C) Model for the role of A3 factors during ribosome assembly. Basepairing between 5.8S and 25S RNAs is indicated by short red lines. Dashed red lines indicate that the basepairing is affected. Dashed black lines indicate that the pre-rRNAs are being turned over. Dashed circles show that the r-proteins might not be tightly associated with preribosomes. (Left panel) Binding of A3 factors (yellow diamonds) determined by CRAC (Granneman et al, 2011) allows basepairing between 5.8S and 25S rRNAs. It also enables stable association of rpL17, rpL26, rpL35, and rpL37 (blue circles), and all proteins together help Rat1 to stop at the B1S site. rpL17 is also required for processing within ITS2. (Right panel) Preribosomes undergo turnover in the absence of A3 factors, and the four r-proteins. Figure source data can be found with the Supplementary Information.
Similar articles
- Ribosomal proteins L7 and L8 function in concert with six A₃ assembly factors to propagate assembly of domains I and II of 25S rRNA in yeast 60S ribosomal subunits.
Jakovljevic J, Ohmayer U, Gamalinda M, Talkish J, Alexander L, Linnemann J, Milkereit P, Woolford JL Jr. Jakovljevic J, et al. RNA. 2012 Oct;18(10):1805-22. doi: 10.1261/rna.032540.112. Epub 2012 Aug 14. RNA. 2012. PMID: 22893726 Free PMC article. - The Role of Ribosomal Proteins eL15 and eL36 in the Early Steps of Yeast 60S Ribosomal Subunit Assembly.
Fernández-Fernández J, Martín-Villanueva S, Perez-Fernandez J, de la Cruz J. Fernández-Fernández J, et al. J Mol Biol. 2023 Dec 15;435(24):168321. doi: 10.1016/j.jmb.2023.168321. Epub 2023 Oct 20. J Mol Biol. 2023. PMID: 37865285 - Immature large ribosomal subunits containing the 7S pre-rRNA can engage in translation in Saccharomyces cerevisiae.
Rodríguez-Galán O, García-Gómez JJ, Kressler D, de la Cruz J. Rodríguez-Galán O, et al. RNA Biol. 2015;12(8):838-46. doi: 10.1080/15476286.2015.1058477. RNA Biol. 2015. PMID: 26151772 Free PMC article. - Principles of 60S ribosomal subunit assembly emerging from recent studies in yeast.
Konikkat S, Woolford JL Jr. Konikkat S, et al. Biochem J. 2017 Jan 15;474(2):195-214. doi: 10.1042/BCJ20160516. Biochem J. 2017. PMID: 28062837 Free PMC article. Review. - Ribosome biogenesis in the yeast Saccharomyces cerevisiae.
Woolford JL Jr, Baserga SJ. Woolford JL Jr, et al. Genetics. 2013 Nov;195(3):643-81. doi: 10.1534/genetics.113.153197. Genetics. 2013. PMID: 24190922 Free PMC article. Review.
Cited by
- Ebp2 and Brx1 function cooperatively in 60S ribosomal subunit assembly in Saccharomyces cerevisiae.
Shimoji K, Jakovljevic J, Tsuchihashi K, Umeki Y, Wan K, Kawasaki S, Talkish J, Woolford JL Jr, Mizuta K. Shimoji K, et al. Nucleic Acids Res. 2012 May;40(10):4574-88. doi: 10.1093/nar/gks057. Epub 2012 Feb 8. Nucleic Acids Res. 2012. PMID: 22319211 Free PMC article. - Analysis of subunit folding contribution of three yeast large ribosomal subunit proteins required for stabilisation and processing of intermediate nuclear rRNA precursors.
Pöll G, Pilsl M, Griesenbeck J, Tschochner H, Milkereit P. Pöll G, et al. PLoS One. 2021 Nov 23;16(11):e0252497. doi: 10.1371/journal.pone.0252497. eCollection 2021. PLoS One. 2021. PMID: 34813592 Free PMC article. - Hierarchical recruitment of ribosomal proteins and assembly factors remodels nucleolar pre-60S ribosomes.
Biedka S, Micic J, Wilson D, Brown H, Diorio-Toth L, Woolford JL Jr. Biedka S, et al. J Cell Biol. 2018 Jul 2;217(7):2503-2518. doi: 10.1083/jcb.201711037. Epub 2018 Apr 24. J Cell Biol. 2018. PMID: 29691304 Free PMC article. - The assembly factor Erb1 functions in multiple remodeling events during 60S ribosomal subunit assembly in S. cerevisiae.
Konikkat S, Biedka S, Woolford JL Jr. Konikkat S, et al. Nucleic Acids Res. 2017 May 5;45(8):4853-4865. doi: 10.1093/nar/gkw1361. Nucleic Acids Res. 2017. PMID: 28115637 Free PMC article. - The RNA recognition motif of NIFK is required for rRNA maturation during cell cycle progression.
Pan WA, Tsai HY, Wang SC, Hsiao M, Wu PY, Tsai MD. Pan WA, et al. RNA Biol. 2015;12(3):255-67. doi: 10.1080/15476286.2015.1017221. RNA Biol. 2015. PMID: 25826659 Free PMC article.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current Protocols in Molecular Biology. John Wiley & Sons Inc.: NY, USA
- Ben-Shem A, Jenner L, Yusupova G, Yusupov M (2010) Crystal structure of the eukaryotic ribosome. Science 330: 1203–1209 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases