Birds as a model to study adult neurogenesis: bridging evolutionary, comparative and neuroethological approaches - PubMed (original) (raw)
Review
Birds as a model to study adult neurogenesis: bridging evolutionary, comparative and neuroethological approaches
Anat Barnea et al. Eur J Neurosci. 2011 Sep.
Abstract
During the last few decades, evidence has demonstrated that adult neurogenesis is a well-preserved feature throughout the animal kingdom. In birds, ongoing neuronal addition occurs rather broadly, to a number of brain regions. This review describes adult avian neurogenesis and neuronal recruitment, discusses factors that regulate these processes, and touches upon the question of their genetic control. Several attributes make birds an extremely advantageous model to study neurogenesis. First, song learning exhibits seasonal variation that is associated with seasonal variation in neuronal turnover in some song control brain nuclei, which seems to be regulated via adult neurogenesis. Second, food-caching birds naturally use memory-dependent behavior in learning the locations of thousands of food caches scattered over their home ranges. In comparison with other birds, food-caching species have relatively enlarged hippocampi with more neurons and intense neurogenesis, which appears to be related to spatial learning. Finally, migratory behavior and naturally occurring social systems in birds also provide opportunities to investigate neurogenesis. This diversity of naturally occurring memory-based behaviors, combined with the fact that birds can be studied both in the wild and in the laboratory, make them ideal for investigation of neural processes underlying learning. This can be done by using various approaches, from evolutionary and comparative to neuroethological and molecular. Finally, we connect the avian arena to a broader view by providing a brief comparative and evolutionary overview of adult neurogenesis and by discussing the possible functional role of the new neurons. We conclude by indicating future directions and possible medical applications.
© 2011 The Authors. European Journal of Neuroscience © 2011 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures
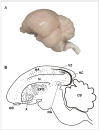
Figure 1
Sagittal views of the avian brain. Rostral is to the right. (A) Side view of an avian brain image (Laughing dove; Streptopelia senegalensis), Photographed by Shay Barkan. (B) Sagittal schematic overview of neurogenesis in the adult avian brain. New neurons are born in the VZ and from there they disperse widely and differentiate into neurons throughout many regions of the forebrain (dots). Regions that incorporate relatively high levels of new neurons are HVC, HC, Area X, N, HA, LPO. No new neurons are incorporated into either RA or the cerebellum (CB). HA -hyperstriatum accessorium, HC - hippocampus, LPO - lobus parolfactorius, N -nidopallium, OB - olfactory bulb, RA - robust nucleus of the arcopallium, VZ -ventricular zone, X - area X. Adapted with permission from Alvarez-Buylla et al., (1994); Doetsch & Scharff (2001).
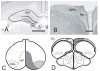
Figure 2
(A, B) Histological comparison of the hippocampal formation between the rat and the pigeon. (A) Layers of the dentate gyrus (DG) and cornu ammonis (CA) are conspicuous in the rat. (B) The V-shaped layer is the only readily apparent structure in the pigeon hippocampus (Hp). APH - area parahippocampalis. (C,D) Location and extent of the pigeon hippocampal formation (HF, dark gray) and dorsolateral corticoid area (CDL, light gray). (C) Dorsal view. (D) Transverse section. Scale bars in A,B = 1 mm. Reproduced with permission from Atoji & Wild (2007) following Rattenborg et al. (2010).
Figure 3
Hypothesized homology between mammalian and avian sub-regions of the hippocampus. Mammalian dentate gyrus is hypothesized to be homologous to avian medioventral V-shaped layer (light grey), mammalian cornu ammonis (CA) and subiculum to avian dorsomedial region (DM), mammalian entorhinal cortex to avian dorsolateral region (DL). Ma - magnocellular region, Pa - parvocellular gerion, Po -cell-poor region, CDL - dorsolateral corticoid area. Reproduced with permission from Atoji & Wild (2007) following Rattenborg et al. (2010).
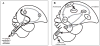
Figure 4
The song control system in birds. (A) The song control system can be imagined as consisting of four modules. Module #1 is in the brain stem and shared by vocal learners as well as by non-vocal learners. Module #2 is a telencephalic module that tells module #1 what to do. Module #3 starts from module #2 and then returns to it; it is necessary for vocal learning but not for production of learned song. Modules #2 and #3 are very well developed in vocal learners, less so or absent in non-learners. Module #4 is the ascending auditory pathway that conveys information about the sounds to be imitated and auditory feedback about the sounds produced. (B) Schematic diagram of the nuclei and connections of modules #2 and #3 and their relation to modules #1 and #4. All the connections shown are ipsilateral and each right and left brain half duplicates the anatomy of the other side. Abbreviations: RA - robust nucleus of archipallium; X - area X of basal ganglia; IMAN - lateral part of the magnocellular nucleus of the anterior nidopallium; DLM - medial portion of dorsolateral thalamic nuclus; nXII - tracheosyringeal part of the hypoglossal nucleus. Field L - auditory nidopallium. Adopted with permission from Nottebohm & Liu (2010).
Figure 5
Examples of doublecortin staining in the hippocampus of free-ranging birds (A) and of captive birds deprived of memory-based experiences (B). (C) A doublecortin stained neuron.
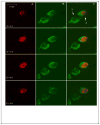
Figure 6
Z-stack images from the HC of European reed warbler (Acrocephalus scirpaceus), under a confocal microscope. Neurons represent only green cytoplasm (labeled by the endogenous marker HU; e.g. cell #1) while new neurons also represent red nuclei (labeled by the birth-date exogenous marker BrdU; cell # 2), these two markers have to co-localize within the same cell along several Z positions. Images were collected at 1.0 μm interval. (A) red 543 nm wavelength frame; (B) green 488nm wavelength frame; (C) combined red and green wavelengths. (Photographed by Shay Barkan).
Similar articles
- Neurogenesis in the adult avian song-control system.
Brenowitz EA, Larson TA. Brenowitz EA, et al. Cold Spring Harb Perspect Biol. 2015 Jun 1;7(6):a019000. doi: 10.1101/cshperspect.a019000. Cold Spring Harb Perspect Biol. 2015. PMID: 26032719 Free PMC article. Review. - Birth, migration, incorporation, and death of vocal control neurons in adult songbirds.
Alvarez-Buylla A, Kirn JR. Alvarez-Buylla A, et al. J Neurobiol. 1997 Nov;33(5):585-601. J Neurobiol. 1997. PMID: 9369461 Review. - Comparative approaches to the avian song system.
Brenowitz EA. Brenowitz EA. J Neurobiol. 1997 Nov;33(5):517-31. doi: 10.1002/(sici)1097-4695(19971105)33:5<517::aid-neu3>3.0.co;2-7. J Neurobiol. 1997. PMID: 9369457 Review. - Interactions between environmental changes and brain plasticity in birds.
Barnea A. Barnea A. Gen Comp Endocrinol. 2009 Sep 1;163(1-2):128-34. doi: 10.1016/j.ygcen.2009.03.031. Epub 2009 Apr 8. Gen Comp Endocrinol. 2009. PMID: 19361509 Review. - Seasonal hippocampal plasticity in food-storing birds.
Sherry DF, Hoshooley JS. Sherry DF, et al. Philos Trans R Soc Lond B Biol Sci. 2010 Mar 27;365(1542):933-43. doi: 10.1098/rstb.2009.0220. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20156817 Free PMC article. Review.
Cited by
- Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies.
Augusto-Oliveira M, Arrifano GPF, Malva JO, Crespo-Lopez ME. Augusto-Oliveira M, et al. Cells. 2019 Feb 5;8(2):125. doi: 10.3390/cells8020125. Cells. 2019. PMID: 30764477 Free PMC article. Review. - Unilateral vocal nerve resection alters neurogenesis in the avian song system in a region-specific manner.
Aronowitz JV, Perez A, O'Brien C, Aziz S, Rodriguez E, Wasner K, Ribeiro S, Green D, Faruk F, Pytte CL. Aronowitz JV, et al. PLoS One. 2021 Aug 31;16(8):e0256709. doi: 10.1371/journal.pone.0256709. eCollection 2021. PLoS One. 2021. PMID: 34464400 Free PMC article. - Effects of psychedelics on neurogenesis and broader neuroplasticity: a systematic review.
Lima da Cruz RV, Leão RN, Moulin TC. Lima da Cruz RV, et al. Mol Med. 2024 Dec 19;30(1):244. doi: 10.1186/s10020-024-01013-4. Mol Med. 2024. PMID: 39701927 Free PMC article. Review. - Food restriction reduces neurogenesis in the avian hippocampal formation.
Robertson BA, Rathbone L, Cirillo G, D'Eath RB, Bateson M, Boswell T, Wilson PW, Dunn IC, Smulders TV. Robertson BA, et al. PLoS One. 2017 Dec 6;12(12):e0189158. doi: 10.1371/journal.pone.0189158. eCollection 2017. PLoS One. 2017. PMID: 29211774 Free PMC article. - Environmental Influences on Neuromorphology in the Non-Native Starling Sturnus vulgaris.
Cardilini APA, Micallef S, Bishop VR, Sherman CDH, Meddle SL, Buchanan KL. Cardilini APA, et al. Brain Behav Evol. 2018;92(1-2):63-70. doi: 10.1159/000491672. Epub 2018 Sep 13. Brain Behav Evol. 2018. PMID: 30212810 Free PMC article.
References
- Adar E, Lotem A, Barnea A. The effect of social environment on singing behavior in the zebra finch (Taeniopygia guttata) and its implication for neuronal recruitment. Behav Brain Res. 2008b;187:178–184. - PubMed
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources