Agent-based dynamic knowledge representation of Pseudomonas aeruginosa virulence activation in the stressed gut: Towards characterizing host-pathogen interactions in gut-derived sepsis - PubMed (original) (raw)
Agent-based dynamic knowledge representation of Pseudomonas aeruginosa virulence activation in the stressed gut: Towards characterizing host-pathogen interactions in gut-derived sepsis
John B Seal et al. Theor Biol Med Model. 2011.
Abstract
Background: There is a growing realization that alterations in host-pathogen interactions (HPI) can generate disease phenotypes without pathogen invasion. The gut represents a prime region where such HPI can arise and manifest. Under normal conditions intestinal microbial communities maintain a stable, mutually beneficial ecosystem. However, host stress can lead to changes in environmental conditions that shift the nature of the host-microbe dialogue, resulting in escalation of virulence expression, immune activation and ultimately systemic disease. Effective modulation of these dynamics requires the ability to characterize the complexity of the HPI, and dynamic computational modeling can aid in this task. Agent-based modeling is a computational method that is suited to representing spatially diverse, dynamical systems. We propose that dynamic knowledge representation of gut HPI with agent-based modeling will aid in the investigation of the pathogenesis of gut-derived sepsis.
Methodology/principal findings: An agent-based model (ABM) of virulence regulation in Pseudomonas aeruginosa was developed by translating bacterial and host cell sense-and-response mechanisms into behavioral rules for computational agents and integrated into a virtual environment representing the host-microbe interface in the gut. The resulting gut milieu ABM (GMABM) was used to: 1) investigate a potential clinically relevant laboratory experimental condition not yet developed--i.e. non-lethal transient segmental intestinal ischemia, 2) examine the sufficiency of existing hypotheses to explain experimental data--i.e. lethality in a model of major surgical insult and stress, and 3) produce behavior to potentially guide future experimental design--i.e. suggested sample points for a potential laboratory model of non-lethal transient intestinal ischemia. Furthermore, hypotheses were generated to explain certain discrepancies between the behaviors of the GMABM and biological experiments, and new investigatory avenues proposed to test those hypotheses.
Conclusions/significance: Agent-based modeling can account for the spatio-temporal dynamics of an HPI, and, even when carried out with a relatively high degree of abstraction, can be useful in the investigation of system-level consequences of putative mechanisms operating at the individual agent level. We suggest that an integrated and iterative heuristic relationship between computational modeling and more traditional laboratory and clinical investigations, with a focus on identifying useful and sufficient degrees of abstraction, will enhance the efficiency and translational productivity of biomedical research.
Figures
Figure 1
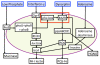
Architecture and topology of the ABM. The ABM simulates the 3-dimensional relationships of the gut-luminal interface by utilizing "stacked" data layers, each one representing a two-dimensional aspect of the gut-microbial interaction environment. It should be noted that the "stacking" occurs only in a virtual sense. This approach is akin to that used in geographical information systems (GIS) [102]. Representative layers depicted include luminal phosphate concentration (green patches), endogenous gut flora population (brown patches), mucous barrier (yellow patches), and epithelial cell tight junctions (violet patches). Agents interact within and between data layers as depicted by Pseudomonas agents (red pentagons) in the mucous and epithelial layers and epithelial cell agents (blue squares) in the epithelial cell layer and interface with the systemic circulation. Simulation world data is passed from one data-layer to the next based on encoded rules in the ABM. Run-time visualization of model layers or variables can be modified at the user interface with application of filters for specific variables to be displayed in the 2-dimensional graphical interface (see Figure 2).
Figure 2
Screenshots of different backgrounds representing data layers. Representative patch backgrounds depicting endogenous gut flora population (brown patches), mucous barrier (yellow patches), epithelial cell tight junctions (violet patches) and epithelial cells (blue GECs on white background). Shading of background color reflects quantitative changes in specific variables (e.g. mucous, endogenous flora, tight junctions). Pseudomonas agents (red pentagons) move to survey microenvironments while epithelial cells (blue squares) modify local conditions in response to host stress. This feature of the ABM aids in initial code development to visually identify encoded behaviors, provides visual reinforcement of expected model behavior and facilitates the use of visual intuition to identify patterns and behaviors that might not be evident in purely tabular data output.
Figure 3
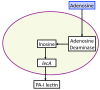
Schematic of P. aeruginosa virulence activation pathway due to adenosine, a host product of ischemia/reperfusion. Intestinal ischemia and reperfusion leads to the production of HIF-1α, which induces the release of adenosine into the intestinal lumen. Adenosine is transported into the bacterial where it is converted to inosine by adenosine deaminase. Inosine induces the expression of the coding region lecA, which is transcribed and translated into the protein PA-I lectin, which is secreted into the intestinal lumen and causes epithelial barrier dysfunction. All the above molecular components are represented by state variables in the GMABM, and the directional arrows indicate the presence of state transition rules.
Figure 4
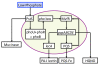
Schematic of P. aeruginosa virulence activation pathways due to bacterial sensing of low phosphate. Low phosphate in the mucous layer of the intestine is sensed by P. aeruginosa through PstS protein. This activation of PstS results in changes in the Pst-PhoU-PhoR complex, leading to histidine kinase PhoR phosphorylation and activation of the transcriptional regulator PhoB that then binds to the pho box gene sequence that controls hundreds of genes including those encoding main regulators of quorum sensing, such as MvfR. MvfR is a transcriptional regulator that acts upstream of the operon pqsABCDE, which codes for, among other things, the enzymes that lead to the production of PQS, a quorum sensing compound, and the bactericidal compound HQNO. PQS serves three additional functions: 1) activates lecA, which leads to the production of PAI-lectin, 2) is secreted to bind to free iron (Fe), and 3) feeds back to enhance the binding of MvfR to the promoter sequence upstream of pqsABCDE. All the above molecular components are represented by state variables in the GMABM, and the directional arrows indicate the presence of state transition rules.
Figure 5
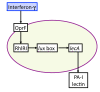
Schematic of P. aeruginosa virulence activation pathways due to interferon-γ, a product of host inflammation. Host cells subject to inflammation secrete the cytokine interferon-γ (IFN-γ). IFN-γ binds to outer membrane porin OprF on P. aeruginosa. Bound OprF activates RhlI, a N-(butanoyl)-L-homoserine lactone synthetase in the quorum sensing system, which in turn is required for PA-I lectin production. All the above molecular components are represented by state variables in the GMABM, and the directional arrows indicate the presence of state transition rules.
Figure 6
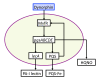
Schematic of P. aeruginosa virulence activation pathways due to bacterial sensing of endogenous opioids, a product of host stress. Endogenous opioids are release by host tissues during systemic stress. Dynorphin, a synthetic agonist used to study opioid receptors, activates the transcriptional regulator MvfR, and leads to the expression of its regulated operon pqsABCDE and subsequent downstream products production of HQNO, and PQS, as noted above in the low phosphate signaling pathways (Figure 4). All the above molecular components are represented by state variables in the GMABM, and the directional arrows indicate the presence of state transition rules.
Figure 7
Schematic of aggregated P. aeruginosa virulence activation pathways associated with host systemic surgical stress. A summary of the four virulence pathways depicted in Figures 3, 4, 5 and 6 is presented in aggregated form. Note the points of convergence and intersections among the different pathways, particularly in terms of downstream effects, suggesting highly conserved and advantageous functions for the virulence outputs of P. aeruginosa. Also note the putative link between low phosphate sensing and opioid sensing reflected by the association between pho box and MvfR (seen in the box outlined in Red). MvfR is clearly an important control point in the phenotypic switching between non-virulent and virulent states, and represents a target for future investigation.
Figure 8
Cross-model validation of virulence expression in Pseudomonas agents to experimental model of transient ischemia. This figure demonstrates adenosine-induced Pseudomonas agent expression of lecA and subsequent production of PA-I lectin. PA-O lectin would then lead to reduced expression of tight junction proteins in GEC agents (see Additional File 2 for sample experimental referent data).
Figure 9
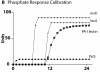
Cross-model validation of virulence expression in Pseudomonas agents to experimental model of low phosphate. These simulations of low phosphate conditions show the results of Pseudomonas agent virulence activation in response to low phosphate sensing, reflected in the production of PstS, MvfR, lecA and PA-I lectin (see Additional File 3 for sample experimental referent data).
Figure 10
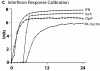
Cross-model validation of virulence expression in Pseudomonas agents to experiments of gut epithelial immune activation. This figure displays the results of simulations of GEC agent production of IFN-γ with binding to Pseudomonas agent surface receptor OprF, and subsequent Pseudomonas agent production of PA-I lectin (see Additional File 4 for experimental referent data).
Figure 11
Cross-model validation of virulence expression in Pseudomonas agents to experiments of endogenous opioid production. This figure demonstrates the results of simulations of the production of endogenous dynorphin by GEC agents in response to a simulation of 20 minutes of ischemia, and the effects of the dynorphin production on Pseudomonas agents' levels of MvfR and HQNO production (see Additional File 5 for sample experimental referent data). Note that there is a discrepancy in the final trajectory of HQNO production between the ABM and the experimental referent. However, the effect of this discrepancy is not apparent in the following figures that demonstrate the suppression of commensal bacterial growth.
Figure 12
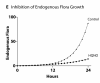
Cross-model validation of virulence expression in Pseudomonas agents in experiments of endogenous opioid production manifesting as suppression of commensal bacterial populations. This figure demonstrates the results of simulations of the production of endogenous dynorphin by GEC agents in response to a simulation of 20 minutes of ischemia, activation of the virulence factor HQNO in the Pseudomonas agents and its effect on the suppression of the growth of commensal bacteria (compare to Additional File 6).
Figure 13
Cross-model validation to experiments of endogenous opioid production concerning the population dynamics of Pseudomonas agents and endogenous flora. The competitive advantage of the Pseudomonas agents is due to the suppression of commensal bacteria resulting from the Pseudomonas agents' activation of virulence factors and production of HQNO (which inhibits commensal bacterial growth) (information extracted from Additional File 6).
Figure 14
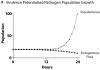
Effect of initial Pseudomonas agent population on simulated host injury. Selected frames from the model interface during simulation of phosphate depletion depict Pseudomonas agents (red pentagons) and tight junctions (purple background), with black background indicating severe barrier disruption. Upper Row of Screenshots: An initial Pseudomonas agent population of 100 agents produced rapid and severe barrier disruption within 12 hours of phosphate depletion and near complete at 36 hours. We considered this to a disproportionally lethal response and non-realistic calibration behavior. Second Row of Screenshots: An initial population of 10 Pseudomonas agents produced moderate injury after 48 hours of phosphate depletion. These dynamics appeared to meet the standard of face validity with respect to the clinically relevant situation, and provided an enhanced ability to identify the properties of the system's tipping point. Graph: These graph demonstrates the relative effect on commensal bacteria and GEC tight junctions at N0 = 100 and 10 respectively.
Figure 15
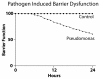
GMABM response to transient intestinal ischemia. The effects of 30 minutes of transient intestinal ischemia, including release of dynorphin and adenosine into the intestinal lumen, were simulated. We utilized two in silico experimental groups: a control group (Pseudomonas agents = 0) and a Pseudomonas group (Pseudomonas agents = 10). Transient ischemia alone produced no significant disruption (Control). The combination of simulated transient intestinal ischemia and the presence of Pseudomonas agents yielded a 40% decrease in barrier function at 24 hours of simulation time. Note that the initiation of the effect can be seen between 6 and 12 hours, suggesting that this period should be targeted for sampling in any future in vivo experiments to obtain the most potentially relevant data.
Figure 16
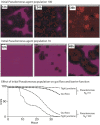
GMABM response to phosphate depletion alone. Selected frames from the model interface taken at 6, 12, 24 and 48 hrs of simulated time during simulation of phosphate depletion in the integrated GMABM. Pseudomonas agents are red pentagons and intact tight junctions are seen as the purple background, with black background indicating severe barrier disruption. Phosphate depletion alone yielded moderate to severe barrier disruption after 48 hours. This response was more severe than expected given prior experimental referent data.
Figure 17
GMABM response to host stress without phosphate depletion. Selected frames from the model interface taken at 6, 12, 24 and 48 hrs of simulated time during simulation of systemic inflammation (IFN-ϒ) without phosphate depletion in the integrated GMABM. Pseudomonas agents are red pentagons and intact tight junctions are seen as the purple background. This set of simulations demonstrate only mild barrier disruption (grey-black areas), consistent with experimental findings that immune activation alone in in vivo models is not sufficient to generate a clinically significant injury.
Figure 18
GMABM response to host stress and phosphate depletion. Selected frames from the model interface taken at 6, 12, 24 and 48 hrs of simulated time during simulation of systemic inflammation (IFN-ϒ) with phosphate depletion. Pseudomonas agents are red pentagons and intact tight junctions are seen as the purple background, with black background indicating severe barrier disruption. Phosphate depletion in conjunction with inflammation combination resulted in the most severe disruption of barrier function. This finding is consistent with that suggested in prior experimental studies.
Figure 19
GMABM response to host stress and low phosphate with respect to Pseudomonas agent and commensal flora population dynamics. The combination of phosphate depletion and inflammation produced the greatest reduction in endogenous flora and increase in Pseudomonas agent population over the course of 48 hours simulated time.
Similar articles
- Agent-based model of epithelial host-pathogen interactions in anastomotic leak.
Stern JR, Olivas AD, Valuckaite V, Zaborina O, Alverdy JC, An G. Stern JR, et al. J Surg Res. 2013 Oct;184(2):730-8. doi: 10.1016/j.jss.2012.12.009. Epub 2013 Jan 1. J Surg Res. 2013. PMID: 23290531 Free PMC article. - Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa.
Alverdy J, Holbrook C, Rocha F, Seiden L, Wu RL, Musch M, Chang E, Ohman D, Suh S. Alverdy J, et al. Ann Surg. 2000 Oct;232(4):480-9. doi: 10.1097/00000658-200010000-00003. Ann Surg. 2000. PMID: 10998646 Free PMC article. - Host stress and virulence expression in intestinal pathogens: development of therapeutic strategies using mice and C. elegans.
Zaborina O, Zaborin A, Romanowski K, Babrowski T, Alverdy J. Zaborina O, et al. Curr Pharm Des. 2011;17(13):1254-60. doi: 10.2174/138161211795703771. Curr Pharm Des. 2011. PMID: 21470113 Free PMC article. Review. - Pseudomonas aeruginosa expresses a lethal virulence determinant, the PA-I lectin/adhesin, in the intestinal tract of a stressed host: the role of epithelia cell contact and molecules of the Quorum Sensing Signaling System.
Wu L, Holbrook C, Zaborina O, Ploplys E, Rocha F, Pelham D, Chang E, Musch M, Alverdy J. Wu L, et al. Ann Surg. 2003 Nov;238(5):754-64. doi: 10.1097/01.sla.0000094551.88143.f8. Ann Surg. 2003. PMID: 14578740 Free PMC article. - Pathogenesis of intestinal Pseudomonas aeruginosa infection in patients with cancer.
Markou P, Apidianakis Y. Markou P, et al. Front Cell Infect Microbiol. 2014 Jan 7;3:115. doi: 10.3389/fcimb.2013.00115. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24432250 Free PMC article. Review. No abstract available.
Cited by
- Host-pathogen interactions between the human innate immune system and Candida albicans-understanding and modeling defense and evasion strategies.
Dühring S, Germerodt S, Skerka C, Zipfel PF, Dandekar T, Schuster S. Dühring S, et al. Front Microbiol. 2015 Jun 30;6:625. doi: 10.3389/fmicb.2015.00625. eCollection 2015. Front Microbiol. 2015. PMID: 26175718 Free PMC article. Review. - Examining the pathogenesis of breast cancer using a novel agent-based model of mammary ductal epithelium dynamics.
Chapa J, Bourgo RJ, Greene GL, Kulkarni S, An G. Chapa J, et al. PLoS One. 2013 May 21;8(5):e64091. doi: 10.1371/journal.pone.0064091. Print 2013. PLoS One. 2013. PMID: 23704974 Free PMC article. - Different Dose-Dependent Modes of Action of C-Type Natriuretic Peptide on Pseudomonas aeruginosa Biofilm Formation.
Desriac F, Clamens T, Rosay T, Rodrigues S, Tahrioui A, Enault J, Roquigny L, Racine PJ, Taupin L, Bazire A, Dufour A, Leprince J, Bouffartigues E, Chevalier S, Feuilloley MGJ, Lesouhaitier O. Desriac F, et al. Pathogens. 2018 Apr 24;7(2):47. doi: 10.3390/pathogens7020047. Pathogens. 2018. PMID: 29695043 Free PMC article. - Agent-Based Modeling of Systemic Inflammation: A Pathway Toward Controlling Sepsis.
An G, Cockrell RC. An G, et al. Methods Mol Biol. 2021;2321:231-257. doi: 10.1007/978-1-0716-1488-4_20. Methods Mol Biol. 2021. PMID: 34048021 - In silico drug absorption tract: An agent-based biomimetic model for human oral drug absorption.
Deng J, Jhandey A, Zhu X, Yang Z, Yik KFP, Zuo Z, Lam TN. Deng J, et al. PLoS One. 2018 Aug 31;13(8):e0203361. doi: 10.1371/journal.pone.0203361. eCollection 2018. PLoS One. 2018. PMID: 30169515 Free PMC article.
References
- Wu L, Holbrook C, Zaborina O, Ploplys E, Rocha F, Pelham D, Chang E, Musch M, Alverdy J. Pseudomonas aeruginosa expresses a lethal virulence determinant, the PA-I lectin/adhesin, in the intestinal tract of a stressed host: the role of epithelia cell contact and molecules of the Quorum Sensing Signaling System. Annals of surgery. 2003;238(5):754–64. doi: 10.1097/01.sla.0000094551.88143.f8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical