Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14 - PubMed (original) (raw)
. 2011 Nov;31(22):4609-22.
doi: 10.1128/MCB.05766-11. Epub 2011 Sep 19.
Kate D Sutherland, François Vaillant, David E Gyorki, Di Wu, Sheridan Holroyd, Kelsey Breslin, Teresa Ward, Wei Shi, Mary L Bath, Siddhartha Deb, Stephen B Fox, Gordon K Smyth, Geoffrey J Lindeman, Jane E Visvader
Affiliations
- PMID: 21930782
- PMCID: PMC3209243
- DOI: 10.1128/MCB.05766-11
Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14
Marie-Liesse Asselin-Labat et al. Mol Cell Biol. 2011 Nov.
Abstract
The transcription factor Gata-3 is a definitive marker of luminal breast cancers and a key regulator of mammary morphogenesis. Here we have explored a role for Gata-3 in tumor initiation and the underlying cellular mechanisms using a mouse model of "luminal-like" cancer. Loss of a single Gata-3 allele markedly accelerated tumor progression in mice carrying the mouse mammary tumor virus promoter-driven polyomavirus middle T antigen (MMTV-PyMT mice), while overexpression of Gata-3 curtailed tumorigenesis. Through the identification of two distinct luminal progenitor cells in the mammary gland, we demonstrate that Gata-3 haplo-insufficiency increases the tumor-initiating capacity of these progenitors but not the stem cell-enriched population. Overexpression of a conditional Gata-3 transgene in the PyMT model promoted cellular differentiation and led to reduced tumor-initiating capacity as well as diminished angiogenesis. Transcript profiling studies identified caspase-14 as a novel downstream target of Gata-3, in keeping with its roles in differentiation and tumorigenesis. A strong association was evident between GATA-3 and caspase-14 expression in preinvasive ductal carcinoma in situ samples, where GATA-3 also displayed prognostic significance. Overall, these studies identify GATA-3 as an important regulator of tumor initiation through its ability to promote the differentiation of committed luminal progenitor cells.
Figures
Fig. 1.
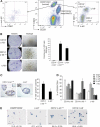
Isolation of a distinct alveolar progenitor cell using the CD14 and c-kit markers. (A) Representative FACS dot plot showing the expression of CD29 and CD24 in Lin− (CD31− CD45− TER119−) cells isolated from 8-week-old virgin mouse mammary glands (middle panel). Representative FACS dot plots showing CD61 and c-kit expression (left panel) or CD14 and c-kit expression (right panel) in Lin− CD29lo CD24+ cells in mammary glands taken from an 8-week-old virgin mouse. (B) Colony-forming capacity of c-kit/CD14 subpopulations plated on fibroblast feeder layers or embedded in Matrigel, as indicated. Right panel, histogram showing the colony formation capacity of c-kit/CD14 cells plated in Matrigel. Data represent the means ± standard error of the means (SEM) of results of three independent experiments. (C) Representative anti-milk protein immunostaining of CD14+ c-kit−/lo and c-kit+ colonies grown in Matrigel for 14 days. Left inset shows staining for the isotype control antibody; right inset shows milk staining of a section from an 18.5-day-pregnant-mouse mammary gland. Scale bars, 60 μm. Right panel, bar chart showing the percentage of milk protein-positive colonies observed 14 days after plating of CD14+ c-kit−/lo and c-kit+ cells in Matrigel (means ± SEM of results of three independent experiments). (D) Histogram showing the percentage of c-kit/CD14 cells in the Lin− CD29lo CD24+ population at different stages of development: virgin (V), pregnancy (dP), and lactation (dL). Results represent the means ± SEM of results for three to five animals per group. (E) β-Galactosidase activity in freshly cytospun CD29hi CD24+ (MaSC-enriched), c-kit+, CD14+ c-kit−/lo, and CD14− c-kit− cells from Gata-3+/nlslacZ mice. Scale bars, 25 μm.
Fig. 2.
Gata-3 is important for mammary stem cell function. (A) Representative whole-mounted outgrowths obtained 8 weeks posttransplantation of CD29hi CD24+ cells from Gata-3+/f or MMTV-cre; Gata-3f/f glands. Scale bars, 1 mm. (B) Bar chart showing percentage of fat pad filling by outgrowths 8 weeks posttransplantation of CD29hi CD24+ cells from Gata-3+/f or MMTV-cre; Gata-3f/f mammary glands.
Fig. 3.
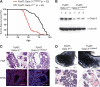
Loss of one Gata-3 allele reduces tumor latency and affects tumor morphology. (A) Kaplan-Meier tumor-free survival curves of PyMT; Gata-3+/+ and PyMT; Gata-3+/nlslacZ mice. The median tumor-free survival times were 78 days versus 48 days, respectively (P < 0.0001). (B) Western blot analysis showing expression level of Gata-3 in three representative PyMT; Gata-3+/+ and PyMT; Gata-3+/nlsLacZ mammary tumors. (C) Hematoxylin and eosin (scale bars, 200 μm) and immunofluorescence staining for NPt2b in mammary lesions arising in 7-week-old PyMT; Gata-3+/+ and PyMT; Gata-3+/nlslacZ mice (scale bars, 100 μm). (D) Whole-mount (scale bars, 2 mm) and hematoxylin and eosin (scale bars, 200 μm) sections of tumors arising from transplanted unsorted cells (160 cells) from 5- to 6-week-old PyMT; Gata-3+/+ and PyMT; Gata-3+/nlslacZ mice.
Fig. 4.
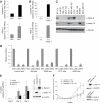
Expansion of luminal progenitor subsets in PyMT; Gata-3+/nlslacZ mammary lesions. (A) Representative FACS dot plot showing the expression of c-kit and CD14 in Lin− CD24+ cells of mammary adenomas (5 to 6 weeks) from PyMT; Gata-3+/+ and PyMT; Gata-3+/nlslacZ mice. (B) Histogram showing percentage of c-kit/CD14 cells in the CD24+ populations isolated from 5- to 6-week-old PyMT; Gata-3+/+ and PyMT; Gata-3+/nlslacZ mice (means ± standard errors of the mean [SEM] of results for five animals per group). (C) Histogram showing fold change (PyMT; Gata-3+/+ over PyMT; Gata-3+/nlslacZ) in tumor-initiating cell (TIC) capacity after transplantation of c-kit+, CD14+ c-kit−/lo, and CD14− c-kit− cells from PyMT; Gata-3+/+ and PyMT; Gata-3+/nlslacZ mice (means ± SEM of results from three independent experiments). *, P = 0.04. Student t test of fold change in TIC capacity for c-kit+ and CD14− c-kit− cells.
Fig. 5.
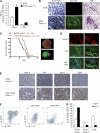
Forced expression of Gata-3 delays mammary tumor formation in MMTV-PyMT mice and induces a differentiated phenotype. (A) Quantitative RT-PCR analysis showing the expression of Gata-3 in freshly sorted CD24+ and CD24− cells from 8-week-old control mice (MTB) and inducible Gata-3 transgenic mice (MTB; Gata-3+/Tg), 4 weeks posttreatment with doxycycline. (B) Whole-mount and mammary gland sections from 7-week-old MTB and MTB; Gata-3+/Tg mice 4 weeks after treatment with doxycycline. Bright-field images show hematoxylin staining of mammary whole mounts. Dark-field images show GFP expression in mammary gland whole mounts. Scale bars, 250 μm. Hematoxylin and eosin staining section: scale bars, 50 μm. (C) Kaplan-Meier plots showing tumor-free survival of PyMT; Gata-3+/+ and PyMT; Gata-3+/Tg mice treated with doxycycline (from 4 weeks of age). Median tumor-free survival times were 66 versus 86 days, respectively (P < 0.05). Right panel: bright-field and dark-field images showing GFP expression in PyMT; MTB; Gata-3+/Tg tumor. Scale bars, 2 mm. (D) GFP expression, DAPI staining, and Gata-3 immunofluorescence staining on consecutive sections of PyMT; MTB; Gata-3+/Tg tumors. Scale bars, 50 μm. (E) Immunohistochemical staining for Gata-3, estrogen receptor α (ERα), cytokeratin 18 (CK18), and smooth muscle actin (SMA) in sections from PyMT; Gata-3+/Tg and PyMT; MTB; Gata-3+/Tg tumors. Scale bars, 50 μm. (F) FACS analysis of PyMT; MTB; Gata-3+/Tg tumors showing the expression of GFP and CD24 in Lin− cells (left panel). Right panel shows the expression of CD14 and c-kit in GFP+ and GFP− cells. (G) Histogram showing percentages of c-kit/CD14 cells in CD24+ GFP+ or CD24+ GFP− subpopulations from PyMT; MTB; Gata-3+/Tg mice (means ± SEM of results for three animals per group).
Fig. 6.
Overexpression of Gata-3 reduces tumor angiogenesis in MMTV-PyMT mice. (A) Representative dot plot showing the expression of GFP and CD31 in PyMT; MTB; Gata-3+/Tg mammary tumors. (B) Scattered plot showing the inverse correlation between CD31 expression and GFP expression in eight individual PyMT; MTB; Gata-3+/Tg mammary tumors.
Fig. 7.
The Gata-3 target gene, caspase-14, promotes differentiation and delays breast tumor formation. (A) Expression analysis of caspase-14 and Gata-3 in GFP+ and GFP− cells from PyMT; MTB; Gata-3+/Tg mammary tumors (means ± standard errors of the mean [SEM] of results for seven tumors per group). (B) Expression analysis of caspase-14 and Gata-3 in immortalized mammary epithelial cells isolated from Gata-3f/f mice, transduced sequentially with pMSCV-cre retrovirus and then Gata-3-expressing (or empty) pBABE retrovirus. (C) Western blot showing the expression of GATA-3, caspase-14, and tubulin in human breast cancer cell lines. (D) ChIP analysis of endogenous GATA-3 binding to four putative GATA-3 binding sites identified within a 10-kb upstream regulatory region of caspase-14 (−484, −3,060, −3,772, and −4,392 from the transcription start site) and a flanking region of caspase-14 promoter with no GATA-3 binding site (control) in MCF-7 cells. Unprecipitated chromatin provided the input control (mean ± SEM of results of three experiments). Mouse IgG and Gata-3 ChIP values for each region were compared using the Student t test: −484 (P = 0.013), −3,060 (P = 0.058), −3,772 (P = 0.028), −4,392 (P = 0.067), control (P = 0.195). (E) β-casein mRNA expression in HC11 cells transduced with control (empty pFU-TA-GFP), pFU-TA-GFP-Gata-3-, or caspase-14-expressing lentiviruses (means ± SEM of results of four independent experiments). Right panel shows Western blot analysis of Gata-3, caspase-14, and β-actin in transduced HC11 cells. (F) Kinetics of tumor formation after orthotopic transplantation of 500,000 MDA-MB-231Luci cells transduced with either pFU-TA-GFP-Gata-3- or caspase-14-expressing lentiviruses or control (vector) virus (means ± SEM of results for six animals per group). Right panel shows Western blot analysis of caspase-14, GATA-3, GAPDH, and tubulin expression in transduced MDA-MB-231Luci cells at the time of transplantation.
Fig. 8.
High GATA-3 levels in human ductal carcinoma in situ (DCIS) are associated with better relapse-free survival. (A) Kaplan-Meier curve showing relapse-free survival in GATA-3-positive and -negative DCIS samples. (B) Representative immunohistostaining for GATA-3 and caspase-14 in human DCIS specimens.
References
- Arnold J. M., et al. 2010. Frequent somatic mutations of GATA3 in non-BRCA1/BRCA2 familial breast tumors, but not in BRCA1-, BRCA2- or sporadic breast tumors. Breast Cancer Res. Treat. 119:491–496 - PubMed
- Asselin-Labat M. L., et al. 2007. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 9:201–209 - PubMed
- Bertucci F., et al. 2000. Gene expression profiling of primary breast carcinomas using arrays of candidate genes. Hum. Mol. Genet. 9:2981–2991 - PubMed
- Bouras T., et al. 2008. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3:429–441 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases