Identification of the earliest natural killer cell-committed progenitor in murine bone marrow - PubMed (original) (raw)
Identification of the earliest natural killer cell-committed progenitor in murine bone marrow
John W Fathman et al. Blood. 2011.
Abstract
Natural killer (NK) cells develop in the bone marrow and are known to gradually acquire the ability to eliminate infected and malignant cells, yet the cellular stages of NK lineage commitment and maturation are incompletely understood. Using 12-color flow cytometry, we identified a novel NK-committed progenitor (pre-NKP) that is a developmental intermediate between the upstream common lymphoid progenitor and the downstream NKP, previously assumed to represent the first stage of NK lineage commitment. Our analysis also refined the purity of NKPs (rNKP) by 6-fold such that 50% of both pre-NKP and rNKP cells gave rise to NKp46+ NK cells at the single-cell level. On transplantation into unconditioned Rag2-/-Il2rγc-/- recipients, both pre-NKPs and rNKPs generated mature NK cells expressing a repertoire of Ly49 family members that degranulated on stimulation ex vivo. Intrathymic injection of these progenitors, however, yielded no NK cells, suggesting a separate origin of thymic NK cells. Unlike the rNKP, the pre-NKP does not express IL-2Rβ (CD122), yet it is lineage committed toward the NK cell fate, adding support to the theory that IL-15 signaling is not required for NK commitment. Taken together, our data provide a high-resolution in vivo analysis of the earliest steps of NK cell commitment and maturation.
Figures
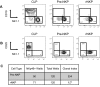
Figure 1
Pre-NKPs and rNKPs can be highly purified from BM using 12-color flow cytometry. (A) Gating strategy for CLP, pre-NKP, and rNKP. (B) Frequency of CLP, pre-NKP, and rNKP in BM isolated from 2 femurs. (C) Absolute number of CLP, pre-NKP, and rNKP from 2 femurs. Shown are the individual data from 10 mice with the mean and standard deviation (B-C).
Figure 2
Pre-NKP and rNKP are lineage restricted and give rise only to NK cells in vitro. (A) Pre-NKP and rNKP lack B-cell potential in vitro. Individual wells with OP9 cells were seeded with 10 indicated progenitor cells and cultured in the presence of SCF, IL-7, Flt3 ligand, and IL-15. Wells were analyzed by flow cytometry 10 days later. No wells were positive for Gr-1+CD11b+ myeloid cells (data not shown). (B) Pre-NKP and rNKP lack in vitro T-cell potential. Flow cytometry analysis of the lineage output of individual wells seeded on OP9-DL1 cells with 10 indicated progenitor cells. No wells analyzed were positive for myeloid cells (data not shown). Data are representative of 1 of 4 experiments. (C) Pre-NKP and rNKP generate NK cells at a high clonal frequency. Number of wells plated with single cell and the number of wells positive for NKp46+ cells after 10 days. Data are representative of 1 of 3 experiments.
Figure 3
Pre-NKP and rNKP are lineage restricted to the NK cell fate in vivo. CD45.1 unfractionated BM, CLP, pre-NKP, and rNKP were transplanted into unconditioned congenic CD45.2 _Rag2_−/−_IL2r_γ_c_−/− recipients. Spleens 3 weeks after transplantation were isolated and analyzed by flow cytometry for lineage potential of donor cells. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 5 experiments.
Figure 4
Pre-NKP and rNKP in vivo–derived NK cells are phenotypically mature and give rise to NK cells on transplantation faster than CLP or LMPP. (A) CD45.1 unfractionated BM, CLP, pre-NKP, and rNKP were transplanted into unconditioned congenic CD45.2 _Rag2_−/−_IL2r_γ_c_−/− recipients. Spleens 3 weeks after transplantation were isolated and analyzed by flow cytometry for mature NK cell markers CD27 and Mac1. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 2 experiments. (B) Ly5.2+ progenitors where transplanted into unconditioned Ly5.1+ DKO mice. Mice where bled every week and analyzed by flow cytometry for donor NK cell reconstitution. Pre-NKP gave rise to NK cells as early as 1 week and were exhausted after 3 weeks. CLP gave rise to few, but detectable NK cells at 1 week and also became exhausted by 4 weeks. We observed no NK cells at 1 week in the LMPP transplants, but by 2 weeks they where comparable to both pre-NKP and CLP. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 2 experiments. (C) Absolute number of donor-derived NK cells from the spleens of mice transplanted with different progenitors. Data are representative of 1 of 2 experiments.
Figure 5
Pre-NKP–derived NK cells have mature functional phenotype. (A) Pre-NKP–derived splenic NK cells express diverse combinations of Ly49 receptors. Wild-type NK cells and donor NKp46+ cells from pre-NKP transplanted _Rag2_−/−_IL2r_γ_c_−/− mice were stained with a panel of anti-Ly49 family antibodies. Data represents 1 of 2 experiments. (B) Pre-NKP–derived NK cells degranulate on activation. Wild-type NK cells and NKp46+ cells derived in vivo from pre-NKP were isolated and stimulated with plate bound anti-NK1.1 for 4 hours in the presence of lysosomal-associated membrane protein 1 (Lamp-1) antibody. Data are representative of 2 independent experiments.
Figure 6
Proposed model for NK bone marrow development. CLP, immature NK cells (iNK), and mature NK cells in the bone marrow, not terminally differentiated splenic NK cells that lack expression of CD27 (mNK).
Similar articles
- FOXO1 and FOXO3 Cooperatively Regulate Innate Lymphoid Cell Development.
Luu TT, Søndergaard JN, Peña-Pérez L, Kharazi S, Krstic A, Meinke S, Schmied L, Frengen N, Heshmati Y, Kierczak M, Bouderlique T, Wagner AK, Gustafsson C, Chambers BJ, Achour A, Kutter C, Höglund P, Månsson R, Kadri N. Luu TT, et al. Front Immunol. 2022 Jun 9;13:854312. doi: 10.3389/fimmu.2022.854312. eCollection 2022. Front Immunol. 2022. PMID: 35757763 Free PMC article. - Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo.
Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Vosshenrich CA, et al. J Immunol. 2005 Feb 1;174(3):1213-21. doi: 10.4049/jimmunol.174.3.1213. J Immunol. 2005. PMID: 15661875 - Identification of the earliest NK-cell precursor in the mouse BM.
Carotta S, Pang SH, Nutt SL, Belz GT. Carotta S, et al. Blood. 2011 May 19;117(20):5449-52. doi: 10.1182/blood-2010-11-318956. Epub 2011 Mar 21. Blood. 2011. PMID: 21422472 - NK Cell Precursors in Human Bone Marrow in Health and Inflammation.
Bozzano F, Perrone C, Moretta L, De Maria A. Bozzano F, et al. Front Immunol. 2019 Aug 28;10:2045. doi: 10.3389/fimmu.2019.02045. eCollection 2019. Front Immunol. 2019. PMID: 31555276 Free PMC article. Review. - Murine natural killer cell progenitors and their requirements for development.
Lian RH, Kumar V. Lian RH, et al. Semin Immunol. 2002 Dec;14(6):453-60. doi: 10.1016/s1044532302000805. Semin Immunol. 2002. PMID: 12457618 Review.
Cited by
- PLZF expression maps the early stages of ILC1 lineage development.
Constantinides MG, Gudjonson H, McDonald BD, Ishizuka IE, Verhoef PA, Dinner AR, Bendelac A. Constantinides MG, et al. Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):5123-8. doi: 10.1073/pnas.1423244112. Epub 2015 Apr 2. Proc Natl Acad Sci U S A. 2015. PMID: 25838284 Free PMC article. - TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy.
Marcoe JP, Lim JR, Schaubert KL, Fodil-Cornu N, Matka M, McCubbrey AL, Farr AR, Vidal SM, Laouar Y. Marcoe JP, et al. Nat Immunol. 2012 Sep;13(9):843-50. doi: 10.1038/ni.2388. Epub 2012 Aug 5. Nat Immunol. 2012. PMID: 22863752 Free PMC article. - Primary Immune Deficiency Treatment Consortium (PIDTC) update.
Griffith LM, Cowan MJ, Notarangelo LD, Kohn DB, Puck JM, Shearer WT, Burroughs LM, Torgerson TR, Decaluwe H, Haddad E; workshop participants. Griffith LM, et al. J Allergy Clin Immunol. 2016 Aug;138(2):375-85. doi: 10.1016/j.jaci.2016.01.051. Epub 2016 Apr 22. J Allergy Clin Immunol. 2016. PMID: 27262745 Free PMC article. Review. - Early events in lymphopoiesis: an update.
Zhang Q, Iida R, Yokota T, Kincade PW. Zhang Q, et al. Curr Opin Hematol. 2013 Jul;20(4):265-72. doi: 10.1097/MOH.0b013e3283612628. Curr Opin Hematol. 2013. PMID: 23594693 Free PMC article. Review. - Fra-2 Is a Dominant Negative Regulator of Natural Killer Cell Development.
Schnoegl D, Hochgerner M, Gotthardt D, Marsh LM. Schnoegl D, et al. Front Immunol. 2022 Jun 22;13:909270. doi: 10.3389/fimmu.2022.909270. eCollection 2022. Front Immunol. 2022. PMID: 35812461 Free PMC article.
References
- Kiessling R, Klein E, Pross H, Wigzell H. ”Natural“ killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5(2):117–121. - PubMed
- Trinchieri G, Santoli D, Dee RR, Knowles BB. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte subpopulation. J Exp Med. 1978;147(5):1299–1313. - PMC - PubMed
- Lanier LL, Phillips JH, Hackett J, Jr, Tutt M, Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986;137(9):2735–2739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01AI047458/AI/NIAID NIH HHS/United States
- T32 AI007290/AI/NIAID NIH HHS/United States
- T32 CA009151/CA/NCI NIH HHS/United States
- R01CA86065/CA/NCI NIH HHS/United States
- R01 AI047457/AI/NIAID NIH HHS/United States
- R01 CA086065/CA/NCI NIH HHS/United States
- 5R01AI047457/AI/NIAID NIH HHS/United States
- F31 AG032854/AG/NIA NIH HHS/United States
- R01 AI047458/AI/NIAID NIH HHS/United States
- T32AI07290/AI/NIAID NIH HHS/United States
- K01 DK078318/DK/NIDDK NIH HHS/United States
- K01DK078318/DK/NIDDK NIH HHS/United States
- CA09151/CA/NCI NIH HHS/United States
- F31AG032854/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources