EphA4 blockers promote axonal regeneration and functional recovery following spinal cord injury in mice - PubMed (original) (raw)
EphA4 blockers promote axonal regeneration and functional recovery following spinal cord injury in mice
Yona Goldshmit et al. PLoS One. 2011.
Abstract
Upregulation and activation of developmental axon guidance molecules, such as semaphorins and members of the Eph receptor tyrosine kinase family and their ligands, the ephrins, play a role in the inhibition of axonal regeneration following injury to the central nervous system. Previously we have demonstrated in a knockout model that axonal regeneration following spinal cord injury is promoted in the absence of the axon guidance protein EphA4. Antagonism of EphA4 was therefore proposed as a potential therapy to promote recovery from spinal cord injury. To further assess this potential, two soluble recombinant blockers of EphA4, unclustered ephrin-A5-Fc and EphA4-Fc, were examined for their ability to promote axonal regeneration and to improve functional outcome following spinal cord hemisection in wildtype mice. A 2-week administration of either of these blockers following spinal cord injury was sufficient to promote substantial axonal regeneration and functional recovery by 5 weeks following injury. Both inhibitors produced a moderate reduction in astrocytic gliosis, indicating that much of the effect of the blockers may be due to promotion of axon growth. These studies provide definitive evidence that soluble inhibitors of EphA4 function offer considerable therapeutic potential for the treatment of spinal cord injury and may have broader potential for the treatment of other central nervous system injuries.
Conflict of interest statement
Competing Interests: The authors have read the journal's policy and have the following conflicts: Martin Pearse is an employee of CSL Limited and who, for the studies described in this manuscript, coordinated the production and quality control of gram quantities of the EphA4-Fc blocker from the EphA4-Fc expression construct as detailed in the methods and provided by Andrew Boyd. Yona Goldshmit, Ann Turnley, Mary Galea, Andrew Boyd and Perry Bartlett have submitted a patent application regarding the use of EphA4 blockers in neural injury, which was sponsored by CSL Ltd (PCT/AU2005/001363). This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
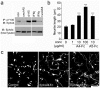
Figure 1. Unclustered ephrin-A5-Fc and EphA4-Fc promote neurite outgrowth.
(A) Immunoprecipitation (IP) of phosphotyrosine containing proteins using anti-pY100 antibody followed by immunoblot (IB) for EphA4 showed that unclustered ephrin-A5-Fc (uncl-A5) inhibits basal and IFNγ-induced EphA4 receptor phosphorylation in cultured astrocytes, whereas clustered ephrin-A5-Fc (cl-A5) upregulates EphA4 receptor phosphorylation. (B–C) Inhibition of neurite outgrowth on astrocytes was blocked in a dose-dependent manner by addition of unclustered EphA4-Fc; 10 µg/ml of EphA4-Fc and ephrin-A5-Fc were used in (C). Results in B show mean±SEM, ***p<0.001, using one-way ANOVA with Tukey's multiple comparison test, from _n_≥100 neurons per condition, representative of n = 3 experiments. Scale bar in C, 100 µm.
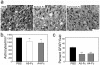
Figure 2. Unclustered ephrin-A5-Fc and EphA4-Fc partially reduce astrocytic gliosis.
(A–C) Immunohistochemical analysis of GFAP expression 4 days after spinal cord hemisection and following 3 days administration of Fc fusion protein indicated that, (A) compared to PBS treatment (n = 6), ephrin-A5-Fc (n = 3) and EphA4-Fc (n = 3) treatment decreased astrocytic gliosis. There were significant decreases in the number of astrocytes (B) and GFAP density at the lesion site was reduced following ephrin-A5-Fc or EphA4-Fc treatment (C). Results in B and C show mean±SEM, **p<0.01, ***p<0.001, using one-way ANOVA with Tukey's multiple comparison test. Scale bar in A, 100 µm.
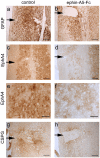
Figure 3. EphA4 immunostaining is decreased adjacent to injury site in ephrin-A5-Fc treated mice.
Immunohistochemical analysis of (A,B) GFAP, (C–F) EphA4 and (G,H) CSPG expression at 2 weeks after spinal cord hemisection and 2 weeks of ephrin-A5-Fc treatment indicated that there was robust GFAP staining in both treated and control mice (A,B). However, in treated mice ipsilateral astrocytes adjacent to the injury site had markedly decreased EphA4 staining (C–F). There was also moderately decreased CSPG staining adjacent to the injury site of treated mice (G,H). Arrows indicate lesion site. Scale bars in A–D, G,H, 100 µm; E,F, 50 µm.
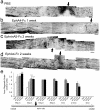
Figure 4. Axonal regrowth towards the lesion site in ephrin-A5-Fc-treated spinal cords 2 weeks after injury.
A montage of confocal images 2 weeks after injury from representative sections anterogradely traced with Fluoro-ruby showing that (A) PBS-treated mice had few labeled axons rostral to the lesion site and that (B) one week of ephrin-A5-Fc treatment increased the number of axons proximal to the lesion site. Axons in the ephrin-A5-Fc-treated mice had robust growth cones. Boxes show enlarged regions in a′ and b′. Scale bars for A, B, A′, B′, 50 µm. Dotted lines in A, B indicate the border of the lesion site and the right hand side of panels is caudal to the lesion site (C) Axonal regrowth was determined by semi-quantitative analysis of axons 100 µm or 750 µm rostral to the lesion site after ephrin-A5-Fc (A5) treatment for 1 week (1 W) or 2 weeks (2 W): ordinal scale 0 = no axons; 1 = fewer than 10 axons; 2 = 10–50 axons; 3 = more than 50 axons per section (from n≥5 sections per spinal cord). *p<0.05, **p<0.01, ***p<0.001 compared to the PBS-treated control, using one-way ANOVA with Tukey's multiple comparison test. (D) Two weeks treatment with ephrin-A5-Fc promoted substantial axonal regeneration into the lesion site; box enlarged in D′. Scale bars D, D′, 50 µm. (E) Regrowth of labeled corticospinal tract axons towards the lesion site at 2 weeks, after 2 weeks EphA4-Fc treatment, with distance from center of injury site; *p<0.05. (F) Representative images of labeled corticospinal tract axons 2 weeks after injury, and a 2-week treatment protocol, showing distance to the center of the injury site.
Figure 5. Axonal regeneration in ephrin-A5-Fc- and EphA4-Fc-treated spinal cords 6 weeks after injury.
A montage of confocal images, 6 weeks after injury, from representative sections anterogradely traced with Fluoro-ruby showing that (A) PBS-treated mice had few labeled axons rostral to the lesion site. (B) One week of EphA4-Fc treatment increased the number of axons rostral to the lesion site but did not promote regeneration through it. A 2-week treatment with ephrin-A5-Fc (C) or EphA4-Fc (D) was sufficient to promote regeneration through and caudal to the lesion site. Scale bars for A–D, 50 µm. Arrows point to center of lesion site. (E) Axonal regrowth was determined by semi-quantitative analysis of labeled axons rostral and caudal to the lesion site after ephrin-A5-Fc (A5) or EphA4-Fc (A4) treatment for 1 week (1 W) or 2 weeks (2 W): ordinal scale 0 = no axons; 1 = fewer than 10 axons; 2 = 10–50 axons; 3 = more than 50 axons per section (from n≥5 sections per spinal cord). *p<0.05, ***p<0.001 compared to PBS control, using one-way ANOVA with Tukey's multiple comparison test.
Figure 6. Ephrin-A5-Fc- or EphA4-Fc-treated mice show significant functional recovery 5 weeks after spinal cord injury.
(A) Walking and climbing on a grid were significantly improved following 2 weeks treatment with ephrin-A5-Fc (A5-2W) or EphA4-Fc (A4-2W). One week of ephrin-A5-Fc (A5-1W) treatment resulted in significant improvement in grid walking but not in climbing as assessed by ANOVA, although it was significant by t-test (mean±SEM, *p<0.05, ***p<0.001 using one-way ANOVA with Tukey's comparison analysis; #p<0.05 using t-test). (B) The mBBB score was measured up to 5 weeks after spinal cord injury (SCI) in PBS- (n = 7) and EphA4-Fc- (A4-Fc) (n = 6) treated mice. Results show mean±SEM (***p<0.001 comparing treatment groups at the indicated time point using one-way ANOVA with Tukey's multiple comparison test). Kinematics of the left hind limb were analyzed from videotapes of locomotion of mice on the treadmill with reflective markers on the iliac crest, hip joint, knee joint and ankle joints. (C–F) Stick figures of the angles between each joint were used to depict one complete step cycle, with the beginning of the swing phase marked with an arrow for the push off point (PO) of the left hind limb and the pattern obtained for a normal intact mouse at a treadmill speed of 12 m/min (C). Panels (D–F) show a representative pattern for one mouse from each of the PBS (n = 7), ephrin-A5-Fc (A5-Fc) (n = 2) and EphA4-Fc (A4-Fc) (n = 6) groups of mice 5 weeks after spinal cord injury, at a treadmill speed of 12 m/min. Treatment over 2 weeks with ephrin-A5-Fc and EphA4-Fc resulted in approximation of the movement pattern seen in intact animals, including a phase of lifting the paw off the surface. (G–I) Average kinematic profile combined from profiles of EphA4-Fc-treated mice (n = 6), of joint angle changes over one complete step cycle of the left hip (G), knee (H) and ankle joints (I) 5 weeks after spinal cord injury in PBS- and EphA4-Fc-treated mice compared to the intact mouse pattern. The point of push off (PO) and the beginning of the swing phase is indicated by a black vertical line through the graph. Results are expressed as mean±SD.
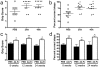
Figure 7. Prolonged treatment with EphA4-Fc and extended recovery.
(A, B) To determine whether extending treatment length to 4 weeks promoted greater functional improvement EphA4-Fc was administered for 2 weeks or 4 weeks, after which (A) grasp score and (B) grid climbing accuracy were assessed at 6 weeks post-injury. Both the 2-week and 4-week treatments improved functional outcomes compared to controls, but were not significantly different to each other. (C, D). To determine whether extending the recovery period for longer than 6 weeks would allow further functional improvement, animals were treated with EphA4-Fc for 2 weeks after injury and assessed at 8, 12 and 24 weeks. Analysis of (C) grasp score and (D) grid climbing accuracy showed that no further improvements were observed after 8 weeks in control or treated mice. (mean±SEM, *p<0.05, **p<0.01).
Similar articles
- Acute delivery of EphA4-Fc improves functional recovery after contusive spinal cord injury in rats.
Spanevello MD, Tajouri SI, Mirciov C, Kurniawan N, Pearse MJ, Fabri LJ, Owczarek CM, Hardy MP, Bradford RA, Ramunno ML, Turnley AM, Ruitenberg MJ, Boyd AW, Bartlett PF. Spanevello MD, et al. J Neurotrauma. 2013 Jun 15;30(12):1023-34. doi: 10.1089/neu.2012.2729. J Neurotrauma. 2013. PMID: 23557244 Free PMC article. - Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice.
Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Goldshmit Y, et al. J Neurosci. 2004 Nov 10;24(45):10064-73. doi: 10.1523/JNEUROSCI.2981-04.2004. J Neurosci. 2004. PMID: 15537875 Free PMC article. - Differential gene expression in the EphA4 knockout spinal cord and analysis of the inflammatory response following spinal cord injury.
Munro KM, Perreau VM, Turnley AM. Munro KM, et al. PLoS One. 2012;7(5):e37635. doi: 10.1371/journal.pone.0037635. Epub 2012 May 22. PLoS One. 2012. PMID: 22629434 Free PMC article. - Role of Axon Guidance Molecules in Ascending and Descending Paths in Spinal Cord Regeneration.
Vartak A, Goyal D, Kumar H. Vartak A, et al. Neuroscience. 2023 Nov 21;533:36-52. doi: 10.1016/j.neuroscience.2023.08.034. Epub 2023 Sep 11. Neuroscience. 2023. PMID: 37704063 Review. - The use of viral vectors to promote repair after spinal cord injury.
Islam A, Tom VJ. Islam A, et al. Exp Neurol. 2022 Aug;354:114102. doi: 10.1016/j.expneurol.2022.114102. Epub 2022 May 2. Exp Neurol. 2022. PMID: 35513025 Free PMC article. Review.
Cited by
- Roles of Eph-Ephrin Signaling in the Eye Lens Cataractogenesis, Biomechanics, and Homeostasis.
Murugan S, Cheng C. Murugan S, et al. Front Cell Dev Biol. 2022 Feb 28;10:852236. doi: 10.3389/fcell.2022.852236. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35295853 Free PMC article. Review. - EphA4 activation of c-Abl mediates synaptic loss and LTP blockade caused by amyloid-β oligomers.
Vargas LM, Leal N, Estrada LD, González A, Serrano F, Araya K, Gysling K, Inestrosa NC, Pasquale EB, Alvarez AR. Vargas LM, et al. PLoS One. 2014 Mar 21;9(3):e92309. doi: 10.1371/journal.pone.0092309. eCollection 2014. PLoS One. 2014. PMID: 24658113 Free PMC article. - Role of EphA4 in Mediating Motor Neuron Death in MND.
Zhao J, Stevens CH, Boyd AW, Ooi L, Bartlett PF. Zhao J, et al. Int J Mol Sci. 2021 Aug 30;22(17):9430. doi: 10.3390/ijms22179430. Int J Mol Sci. 2021. PMID: 34502339 Free PMC article. Review. - Age-dependent transcriptome and proteome following transection of neonatal spinal cord of Monodelphis domestica (South American grey short-tailed opossum).
Saunders NR, Noor NM, Dziegielewska KM, Wheaton BJ, Liddelow SA, Steer DL, Ek CJ, Habgood MD, Wakefield MJ, Lindsay H, Truettner J, Miller RD, Smith AI, Dietrich WD. Saunders NR, et al. PLoS One. 2014 Jun 10;9(6):e99080. doi: 10.1371/journal.pone.0099080. eCollection 2014. PLoS One. 2014. PMID: 24914927 Free PMC article. - EPHA4 is a disease modifier of amyotrophic lateral sclerosis in animal models and in humans.
Van Hoecke A, Schoonaert L, Lemmens R, Timmers M, Staats KA, Laird AS, Peeters E, Philips T, Goris A, Dubois B, Andersen PM, Al-Chalabi A, Thijs V, Turnley AM, van Vught PW, Veldink JH, Hardiman O, Van Den Bosch L, Gonzalez-Perez P, Van Damme P, Brown RH Jr, van den Berg LH, Robberecht W. Van Hoecke A, et al. Nat Med. 2012 Sep;18(9):1418-22. doi: 10.1038/nm.2901. Nat Med. 2012. PMID: 22922411
References
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. - PubMed
- Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr Biol. 2005;15:R749–753. - PubMed
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous