Mitochondrial dysfunction and accumulation of the β-secretase-cleaved C-terminal fragment of APP in Alzheimer's disease transgenic mice - PubMed (original) (raw)
Mitochondrial dysfunction and accumulation of the β-secretase-cleaved C-terminal fragment of APP in Alzheimer's disease transgenic mice
Latha Devi et al. Neurobiol Dis. 2012 Jan.
Abstract
Mitochondrial dysfunction is an early feature of Alzheimer's disease (AD) and may play an important role in the pathogenesis of disease. Emerging evidence indicates that amyloid-β (Aβ) peptides enter mitochondria and may thereby disrupt mitochondrial function in brains of AD patients and transgenic model mice. However, it remains to be determined whether the β-cleaved C-terminal fragment (C99), another neurotoxic fragment of amyloid precursor protein (APP), may accumulate in mitochondria of neurons affected by AD. Using immunoblotting, digitonin fractionation and immunofluorescence labeling techniques, we found that C99 is targeted to mitochondria, in particular, to the mitoplast (i.e., inner membrane and matrix compartments) in brains of AD transgenic mice (5XFAD model). Furthermore, full-length APP (fl-APP) was also identified in mitochondrial fractions of 5XFAD mice. Remarkably, partial deletion of the β-site APP-cleaving enzyme 1 (BACE1(+/-)) almost completely abolished mitochondrial targeting of C99 and fl-APP in 5XFAD mice at 6 months of age. However, substantial amounts of C99 and fl-APP accumulation remained in mitochondria of 12-month-old BACE1(+/-)·5XFAD mouse brains. Consistent with these changes in mitochondrial C99/fl-APP levels, BACE1(+/-) deletion age-dependently rescued mitochondrial dysfunction in 5XFAD mice, as assessed by cytochrome c release from mitochondria, reduced redox or complex activities and oxidative DNA damage. Moreover, BACE1(+/-) deletion also improved memory deficits as tested by the spontaneous alternation Y-maze task in 5XFAD mice at 6 months but not at 12 months of age. Taken together, our findings suggest that mitochondrial accumulation of C99 and fl-APP may occur through BACE1-dependent mechanisms and contribute to inducing mitochondrial dysfunction and cognitive impairments associated with AD.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
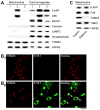
Fig. 1
Mitochondrial accumulation of C99 and fl-APP in 5XFAD mice at 6 months of age. (A) Immunoblot analysis of C99, fl-APP, BACE1 and a set of subcellular marker proteins in mitochondrial fractions and whole brain homogenates from wild-type (1), 5XFAD (2) and BACE1+/−·5XFAD (3) mice. Note that BACE1+/− deletion almost completely abolishes mitochondrial accumulation of C99 and fl-APP in 5XFAD mouse brains. (B) Double immunofluorescence labeling with anti-TOM20 (mitochondrial marker: red) and C1/6.1 antibody against the C-terminus of APP (C99 and fl-APP: green) in the cerebral cortex of wild-type (B1) and 5XFAD (B2) mice. The C1/6.1 immunoreactivity is found intraneuronally or associated with amyloid plaques (asterisk). Note the high degree of colocalization of TOM20 and C1/6.1 immunoreactivities in neurons of 5XFAD mice (white arrows). Scale bar = 20 μm. (C) Immunoblot analysis of C99, fl-APP and mitochondrial marker proteins in mitochondrial fractions of 5XFAD mice that received limited trypsin digestion or digitonin fractionation. Note that both C99 and fl-APP are found primarily in mitochondrial fractions containing the inner membrane and matrix.
Fig. 2
Mitochondrial accumulation of C99 and fl-APP in 5XFAD mice at 12 months of age. (A) Immunoblot analysis of C99, fl-APP and mitochondrial marker proteins in mitochondrial fractions from wild-type (1), 5XFAD (2) and BACE1+/−·5XFAD (3) mouse brains. Note that substantial amounts of C99 and fl-APP accumulation are found in mitochondria of BACE1+/−·5XFAD mouse brains at this advanced age. (B) Limited trypsin digestion and digitonin fractionation show that mitochondrial C99 and fl-APP in 5XFAD mice are primarily associated with the inner membrane-matrix compartments.
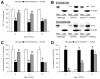
Fig. 3
Age-dependent effects of BACE1+/− deletion on mitochondrial dysfunction in 5XFAD mice. (A) MTT assays revealed that BACE1+/− deletion rescues reduced mitochondrial redox activities in 5XFAD mice at 6 and 9 months but not at 12 months of age (n = 5–8 mice per group). (B) Immunoblot analysis of cytochrome c (cyt c) in mitochondrial (Mito) and cytosolic (Cyto) fractions from wild-type, 5XFAD and BACE1+/−·5XFAD mouse brains. (C, D) Immunoreactive bands for cyt c in mitochondrial (C) and cytosolic (D) fractions were quantified and expressed as percentage of wild-type control and 5XFAD levels, respectively (n = 3–8 mice per group). Note that BACE1+/− deletion rescues cytochrome c release from mitochondria to cytosol in 5XFAD mice at 6 and 9 months but not at 12 months of age. * p < 0.05 (vs. wild-type), # p < 0.05 (vs. 5XFAD). All data are presented as mean ± SEM.
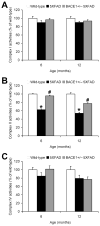
Fig. 4
Age-dependent effects of BACE1+/− deletion on reduced mitochondrial complex activities in 5XFAD mice. While complex I activities (A) are not affected, significant reductions in complex II activities (B) and a trend toward reductions in complex IV activities (C) (p = 0.14 for 6 months, p = 0.06 for 12 months of age) are observed in mitochondria of 5XFAD mice compared with wild-type controls. Note that complex II and IV activities are restored back to wild-type levels in 6-month-old BACE1+/−·5XFAD mice, whereas 12-month-old BACE1+/−·5XFAD mice show only partial or no rescue in these complex activities (n = 5–12 mice per group). * p < 0.05 (vs. wild-type), # p < 0.05 (vs. 5XFAD). All data are presented as mean ± SEM.
Fig. 5
Effects of BACE1+/− deletion on the oxidative DNA damage in 5XFAD mice. Immunofluorescence labeling with anti-8-OH-DG antibody in the cerebral cortex of wild-type (A), 5XFAD (B) and BACE1+/−·5XFAD (C) mice at 6 months of age. Note that 5XFAD mice show a dramatic elevation in the 8-OH-DG immunoreactivity (oxidative DNA damage marker), while it was reduced to wild-type control levels in BACE1+/−·5XFAD mice. Scale bar = 50 μm.
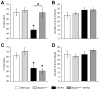
Fig. 6
Age-dependent effects of BACE1+/− deletion on memory deficits in 5XFAD mice. Mice at 6 months (A, B) and 12 months (C, D) of age were tested for memory using the spontaneous alternation Y-maze paradigm. (A, C) Spatial working memory, as assessed by the spontaneous alternation performance, is significantly impaired (around 50% chance levels) in 5XFAD mice irrespective of age compared to wild-type controls (* p < 0.05). Note that BACE1+/−·5XFAD mice are rescued almost completely back to wild-type levels of alternation performance at 6 months but not at 12 months of age (# p < 0.05 versus age-matched 5XFAD). n = 5–10 mice per group. (B, D) Total number of arm entries reflecting exploratory activities of mice in the Y-maze does not differ between four groups irrespective of age, suggesting that the changes are memory-specific. n = 5–10 mice per group. All data are presented as mean ± SEM.
Similar articles
- Effects of BACE1 haploinsufficiency on APP processing and Aβ concentrations in male and female 5XFAD Alzheimer mice at different disease stages.
Devi L, Ohno M. Devi L, et al. Neuroscience. 2015 Oct 29;307:128-37. doi: 10.1016/j.neuroscience.2015.08.037. Epub 2015 Aug 24. Neuroscience. 2015. PMID: 26314636 Free PMC article. - Partial reduction of BACE1 improves synaptic plasticity, recent and remote memories in Alzheimer's disease transgenic mice.
Kimura R, Devi L, Ohno M. Kimura R, et al. J Neurochem. 2010 Apr;113(1):248-61. doi: 10.1111/j.1471-4159.2010.06608.x. Epub 2010 Jan 20. J Neurochem. 2010. PMID: 20089133 Free PMC article. - The Swedish dilemma - the almost exclusive use of APPswe-based mouse models impedes adequate evaluation of alternative β-secretases.
Armbrust F, Bickenbach K, Marengo L, Pietrzik C, Becker-Pauly C. Armbrust F, et al. Biochim Biophys Acta Mol Cell Res. 2022 Mar;1869(3):119164. doi: 10.1016/j.bbamcr.2021.119164. Epub 2021 Oct 23. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 34699873 Review.
Cited by
- The mitochondrial epigenome: a role in Alzheimer's disease?
Devall M, Mill J, Lunnon K. Devall M, et al. Epigenomics. 2014;6(6):665-75. doi: 10.2217/epi.14.50. Epigenomics. 2014. PMID: 25531259 Free PMC article. Review. - RNA silencing of genes involved in Alzheimer's disease enhances mitochondrial function and synaptic activity.
Manczak M, Reddy PH. Manczak M, et al. Biochim Biophys Acta. 2013 Dec;1832(12):2368-78. doi: 10.1016/j.bbadis.2013.09.008. Epub 2013 Sep 21. Biochim Biophys Acta. 2013. PMID: 24063855 Free PMC article. - Biochemical assessment of precuneus and posterior cingulate gyrus in the context of brain aging and Alzheimer's disease.
Maarouf CL, Kokjohn TA, Walker DG, Whiteside CM, Kalback WM, Whetzel A, Sue LI, Serrano G, Jacobson SA, Sabbagh MN, Reiman EM, Beach TG, Roher AE. Maarouf CL, et al. PLoS One. 2014 Aug 28;9(8):e105784. doi: 10.1371/journal.pone.0105784. eCollection 2014. PLoS One. 2014. PMID: 25166759 Free PMC article. - Molecular Dysfunctions of Mitochondria-Associated Membranes (MAMs) in Alzheimer's Disease.
Eysert F, Kinoshita PF, Mary A, Vaillant-Beuchot L, Checler F, Chami M. Eysert F, et al. Int J Mol Sci. 2020 Dec 14;21(24):9521. doi: 10.3390/ijms21249521. Int J Mol Sci. 2020. PMID: 33327665 Free PMC article. Review. - Advancing Alzheimer's Disease Modelling by Developing a Refined Biomimetic Brain Microenvironment for Facilitating High-Throughput Screening of Pharmacological Treatment Strategies.
Mohd Murshid N, Mohd Sahardi NFN, Makpol S. Mohd Murshid N, et al. Int J Mol Sci. 2024 Dec 30;26(1):241. doi: 10.3390/ijms26010241. Int J Mol Sci. 2024. PMID: 39796097 Free PMC article. Review.
References
- Berger-Sweeney J, et al. Impairments in learning and memory accompanied by neurodegeneration in mice transgenic for the carboxyl-terminus of the amyloid precursor protein. Brain Res Mol Brain Res. 1999;66:150–162. - PubMed
- Brown MR, et al. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. - PubMed
- Cai H, et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases