The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: Going round in circles? - PubMed (original) (raw)
Review
The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: Going round in circles?
Mary C Sugden et al. Islets. 2011 Nov-Dec.
Abstract
Pyruvate is the major product of glycolysis in pancreatic β-cells, and its ultimate metabolic fate depends on the relative activities of two enzymes. The first, pyruvate carboxylase (PC) replenishes oxaloacetate withdrawn from the tricarboxylic acid (TCA) cycle via the carboxylation of pyruvate to form oxaloacetate. Flux via PC is also involved in the formation of NADPH, one of several important coupling factors for insulin secretion. In most tissues, PC activity is enhanced by increased acetyl-CoA. The alternative fate of pyruvate is its oxidative decarboxylation to form acetyl-CoA via the pyruvate dehydrogenase complex (PDC). The ultimate fate of acetyl-CoA carbon is oxidation to CO2 via the TCA cycle, and so the PDC reaction results of the irreversible loss of glucose-derived carbon. Thus, PDC activity is stringently regulated. The mechanisms controlling PDC activity include end-product inhibition by increased acetyl-CoA, NADH and ATP, and its phosphorylation (inactivation) by a family of pyruvate dehydrogenase kinases (PDHKs 1-4). Here we review new developments in the regulation of the activities and expression of PC, PDC and the PDHKs in the pancreatic islet in relation to islet pyruvate disposition and glucose-stimulated insulin secretion (GSIS).
Figures
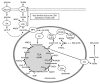
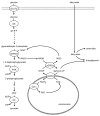
Figure 1. Overview of non-oxidative and oxidative glucose metabolism in islets. By virtue of very low expression and activity of lactate dehydrogenase, pyruvate is the main end product of glycolysis in pancreatic β-cells. Μore than 80% of glucose carbons within the β-cell are oxidized to CO2, which occurs predominantly via the pyruvate dehydrogenase complex and the tricarboxylic acid cycle. Oxaloacetate can be generated from pyruvate via its ATP-dependent carboxylation by pyruvate carboxylase. DIC, dicarboxylate carrier; GDH, glutamate dehydrogenase; GLUT2, glucose transporter 2; GK, glucokinase; LDH, lactate dehydrogenase; MCT, monocarboxylic acid transporter; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; PDHK, pyruvate dehydrogenase kinase.
Figure 2. Regulation of pyruvate dehydrogenase activity. The mechanisms that control PDC activity include end product inhibition by increased mitochondrial acetyl-CoA, NADH and ATP concentrations (which can also be generated by FA oxidation) and post-translational modification, namely its phosphorylation (inactivation) by a family of pyruvate dehydrogenase kinases (PDHKs 1–4). Phosphorylation of site 1 modulates the percentage of active PDC, whereas phosphorylation of sites 2 and 3 may retard reactivation by dephosphorylation by the pyruvate dehydrogenase phosphatases.- All of the PDHKs phosphorylate sites 1 and 2, whereas site 3 is phosphorylated only by PDHK1. Allosteric activation (arrow) and inhibition (blunt end) is indicated by dotted lines. GK, glucokinase; GLUT, glucose transporter; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; PDHK, pyruvate dehydrogenase kinase.
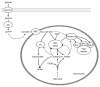
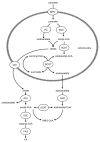
Figure 3. Generation of cytosolic NADPH by the pyruvate/malate pathway. PC catalyzes the conversion of pyruvate to oxaloacetate, which can yield malate catalyzed by mitochondrial MDH. Malate export into the cytosol via the dicarboxylate carrier can lead to the formation of the coupling factor NADPH through the conversion of malate to pyruvate by cytosolic ME. Pyruvate can then re-enter the mitochondria for conversion to oxaloacetate. Critically, while the cME is known to generate NAPDH, the isoform expressed in the mitochondrion (mME) generates NADH. Thus, it is crucial that the TCA cycle intermediates are exported to the cytosol for the formation of NADPH for insulin secretion. DIC, dicarboxylate carrier; MDH, malate dehydrogenase; ME, malic enzyme; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex.
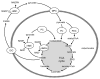
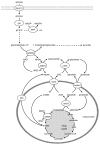
Figure 4. The pyruvate/citrate and pyruvate/isocitrate pathways are initiated by the conversion of pyruvate to both oxaloacetate (via PC) and acetyl-CoA (via PDC), allowing the formation of citrate and subsequently isocitrate. Both citrate and isocitrate can be transported to the cytosol by the citrate-isocitrate carrier (CIC), where citrate can be converted to isocitrate by cytosolic aconitase. Cytosolic citrate can be cleaved by ATP-citrate lyase (ACL) to acetyl-CoA and oxaloacetate. Oxaloacetate can be converted to malate by cytosolic MDH (cMDH), also generating NADH, and then the coupling factor NADPH can be generated by conversion of malate to pyruvate by cME (as in the pyruvate/malate pathway). Pyruvate can then re-enter the mitochondria completing the cycle. Since the pyruvate/citrate cycle also forms cytosolic acetyl-CoA, it can generate malonyl-CoA. Malonyl-CoA serves both as an intermediate in FA synthesis and also a potent inhibitor of FA oxidation at the level of carnitine palmitoyltransferase (CPT) 1. Cytosolic isocitrate can directly yield NADPH by its conversion to 2OG by cICDH. Potentially, 2OG can be converted to oxaloacetate by cAAT, thereby “joining” the pyruvate/citrate cycle, generating NADPH a second time at the level of cME or alternatively, enter the mitochondria via OGC and join the TCA cycle, at the step subsequent to mICDH, and be metabolized to oxaloacetate. AAT, aspartate aminotransferase; ACC, acetyl-CoA carboxylase; ACL, ATP-citrate lyase; CIC, citrate isocitrate carrier; ICDH, isocitrate dehydrogenase; MDH, malate dehydrogenase; ME, malic enzyme; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; OGC, 2-oxoglutarate carrier.
Figure 5. The role of the glycerol-3-phosphate shuttle in NADH regeneration and triacylglycerol (TAG) formation to combat lipotoxicity. Cytoplasmic cGPDH catalyzes the conversion of dihydroxyacetone phosphate (DHAP), generated at the glyceraldehyde phosphate dehydrogenase step of glycolysis, to glycerol-3-phosphate and generates NAD+, which facilitates continued ATP and pyruvate production by glycolysis. Mitochondrial GPDH (mGPDH) converts glycerol-3-phosphate back to DHAP and also generates FADH2, which generates mitochondrial ATP to help fuel exocytosis. The esterification of fatty acids to triacylglycerol also utilizes glycerol-3-phosphate, which may act to combat lipotoxicity, thereby limiting the generation of cytotoxic lipids such as ceramide. DHAP, dihydroxyacetone phosphate; GK, glucokinase; GLUT, glucose transporter; GPDH, glycerol-3-phosphate dehydrogenase; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; PGK, phosphoglycerate kinase; PK, pyruvate kinase.
Figure 6. The role of the malate-aspartate shuttle in NADH regeneration. The malate/aspartate shuttle appears to compensate for the lack of glycerol-3-phosphate shuttling. NADH is transported into the mitochondrion by the interconversion of malate and aspartate via oxaloacetate in the cytoplasm and the mitochondrion and involves cytoplasmic and mitochondrial glutamate and 2-oxoglutarate and isoenzymes of aspartate aminotransferase. The net redox effect of the malate-aspartate shuttle is that NADH in the cytosol is oxidized to NAD+, and NAD+ in the matrix is reduced to NADH. AAT, aspartate aminotransferase; GK, glucokinase; GLUT, glucose transporter; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; MDH, malate dehydrogenase; 2OG, 2-oxoglutarate; OGC, 2-oxoglutarate carrier; PPP, pentose phosphate pathway.
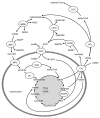
Figure 7. Role of a novel pathway of acyl-CoA synthesis in human islets. Human islets are characterized by elevated succinyl-CoA:3-ketoacid-CoA transferase (SCOT) and acetoacetyl-CoA synthetase (AAS), which form mitochondrial acetoacetate and permit the formation of cytosolic acyl-CoA respectively. This pathway may be more active in human compared with rodent β-cells due to lower pyruvate carboxylation as a result of lowered PC expression and activity (and thus greater pyruvate decarboxylation via PDC) compared with rodent islets. ACAT, acetyl-CoA acetyltransferase; ACC, acetyl-CoA carboxylase; ACL, ATP-citrate lyase; CIC, citrate isocitrate carrier; FAS, fatty acid synthase; MPC, mitochondrial pyruvate carrier; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; SCOT, succinyl-CoA:3-ketoacid-CoA transferase.
Similar articles
- Cardiolipin-induced activation of pyruvate dehydrogenase links mitochondrial lipid biosynthesis to TCA cycle function.
Li Y, Lou W, Raja V, Denis S, Yu W, Schmidtke MW, Reynolds CA, Schlame M, Houtkooper RH, Greenberg ML. Li Y, et al. J Biol Chem. 2019 Jul 26;294(30):11568-11578. doi: 10.1074/jbc.RA119.009037. Epub 2019 Jun 11. J Biol Chem. 2019. PMID: 31186346 Free PMC article. - Lipoyl domain-based mechanism for the integrated feedback control of the pyruvate dehydrogenase complex by enhancement of pyruvate dehydrogenase kinase activity.
Ravindran S, Radke GA, Guest JR, Roche TE. Ravindran S, et al. J Biol Chem. 1996 Jan 12;271(2):653-62. doi: 10.1074/jbc.271.2.653. J Biol Chem. 1996. PMID: 8557670 - Therapeutic potential of the mammalian pyruvate dehydrogenase kinases in the prevention of hyperglycaemia.
Sugden MC, Holness MJ. Sugden MC, et al. Curr Drug Targets Immune Endocr Metabol Disord. 2002 Jul;2(2):151-65. Curr Drug Targets Immune Endocr Metabol Disord. 2002. PMID: 12476789 Review. - Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer.
Stacpoole PW. Stacpoole PW. J Natl Cancer Inst. 2017 Nov 1;109(11). doi: 10.1093/jnci/djx071. J Natl Cancer Inst. 2017. PMID: 29059435 Review.
Cited by
- ROCK1 regulates insulin secretion from β-cells.
Sung BJ, Lim SB, Yang WM, Kim JH, Kulkarni RN, Kim YB, Lee MK. Sung BJ, et al. Mol Metab. 2022 Dec;66:101625. doi: 10.1016/j.molmet.2022.101625. Epub 2022 Oct 29. Mol Metab. 2022. PMID: 36374631 Free PMC article. - Regulation of Mitochondrial Function by Epac2 Contributes to Acute Inflammatory Hyperalgesia.
Goode DJ, Molliver DC. Goode DJ, et al. J Neurosci. 2021 Mar 31;41(13):2883-2898. doi: 10.1523/JNEUROSCI.2368-20.2021. Epub 2021 Feb 16. J Neurosci. 2021. PMID: 33593853 Free PMC article. - The Illustration of Altered Glucose Dependency in Drug-Resistant Cancer Cells.
Bishayee K, Lee SH, Park YS. Bishayee K, et al. Int J Mol Sci. 2023 Sep 11;24(18):13928. doi: 10.3390/ijms241813928. Int J Mol Sci. 2023. PMID: 37762231 Free PMC article. Review. - Roles of Pyruvate, NADH, and Mitochondrial Complex I in Redox Balance and Imbalance in β Cell Function and Dysfunction.
Luo X, Li R, Yan LJ. Luo X, et al. J Diabetes Res. 2015;2015:512618. doi: 10.1155/2015/512618. Epub 2015 Oct 19. J Diabetes Res. 2015. PMID: 26568959 Free PMC article. Review. - Modulation of serotonin signaling by the putative oxaloacetate decarboxylase FAHD-1 in Caenorhabditis elegans.
Baraldo G, Etemad S, Weiss AKH, Jansen-Dürr P, Mack HID. Baraldo G, et al. PLoS One. 2019 Aug 14;14(8):e0220434. doi: 10.1371/journal.pone.0220434. eCollection 2019. PLoS One. 2019. PMID: 31412049 Free PMC article.
References
- Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, Tamarit-Rodriguez J, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem. 1994;269:4895–902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources