Functional analysis of interaction sites on the N-terminal domain of clathrin heavy chain - PubMed (original) (raw)
Functional analysis of interaction sites on the N-terminal domain of clathrin heavy chain
Anna K Willox et al. Traffic. 2012 Jan.
Abstract
In clathrin-mediated membrane traffic, clathrin does not bind directly to cargo and instead binds to adaptors that mediate this function. For endocytosis, the main adaptor is the adaptor protein (AP)-2 complex, but it is uncertain how clathrin contacts AP-2. Here we tested in human cells the importance of the three binding sites that have been identified so far on the N-terminal domain (NTD) of clathrin. We find that mutation of each of the three sites on the NTD, alone or in combination, does not block clathrin/AP-2-mediated endocytosis in the same way as deletion of the NTD. We report here the fourth and final site on the NTD that is required for clathrin/AP-2-mediated endocytic function. Each of the four interaction sites can operate alone to mediate endocytosis. The observed functional redundancy between interaction sites on the NTD explains how productivity of clathrin-coated vesicle formation is ensured.
© 2011 John Wiley & Sons A/S.
Figures
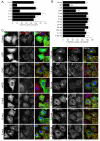
Figure 1. Clathrin lacking binding sites for clathrin-box, W-box and a2L motifs is functional for clathrin/AP-2-mediated endocytosis
A) Rescue of transferrin uptake by CHC with single-site mutations. Bar chart of transferrin uptake in HEK293 cells, normalized to CHC, measured by confocal microscopy. Ncell = 29-87, Nexp = 2. B-C) Rescue of transferrin uptake by CHC with single-, double- or triple-site mutations. B) Bar charts of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 4 (5 for C+ and C+DE). **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. In all figures, ‘Control’ is GFP expressed on a control RNAi background and all other constructs are expressed in CHC RNAi cells. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
Figure 2. The first 100 residues of human CHC are sufficient for clathrin/AP-2-mediated endocytosis
A) Schematic illustration of yeast-human chimeric CHCs (residues 1-330). Human and yeast clathrin are colored pale yellow and green, respectively. Beta-propeller blades are numbered for CHC to aid orientation. B-C) CHC with NTD from chc1p cannot rescue transferrin uptake and uptake depends on residues 1-100. B) Bar charts of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 3. *, p <0.05. **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing chimeric GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
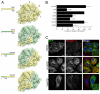
Figure 3. A fourth interaction site on the NTD, defined by sequence conservation, is required for clathrin/AP-2-mediated endocytosis
A) Evolutionary conservation of residues on the surface of the NTD of CHC. (i) Ribbon diagram to show the approximate positions of the three binding sites (PDB codes = 1UTC, 1C9I and 3GD1). (ii) Molecular surface of the NTD with projected sequence conservation colored from cyan (divergent) to maroon (conserved). Ligands are removed in this panel. (iii) Binding sites fall within conserved patches and the back face of the NTD is more divergent. (iv) View of two conserved patches between on blades 6 and 7 suggestive of a new interaction site. (v) Design of two new mutants to target the putative interaction site. B-C) Rescue of transferrin uptake by a triple-site mutant CHC (CDE) with additional mutations at N296A, R297E (CDEF) and at E11K (CDEG). B) Bar charts of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 4. *, p <0.05. **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
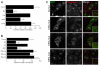
Figure 4. The E11K mutation does not affect clathrin stability and the four interaction sites can each mediate endocytosis alone
A) Rescue of transferrin uptake by a CHC with a single E11K mutation (G). B) Rescue of transferrin uptake by CHCs with wild type binding sites for a2L motifs (CDG), W-box motifs (CEG) and CBMs (DEG) alone. The functionality of the fourth site is shown in Fig 1 (CDE). Bar charts of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 3. **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
Figure 5. Further definition of the fourth interaction site by new inactivating mutations
A) Designing a new inactivating mutation of the fourth site. View of the fourth site (as seen in Fig 3A) showing the new G′ mutation (red) the previous E11K (G mutation) is shown in brown. A sequence alignment is shown for CHCs from different organisms (right). The G′ mutation alters the human residues at positions 14, 16 and 17 to their chc1p counterparts. B) Lack of rescue of transferrin uptake by a CHC with CDEG′ and CDEG. CHC with the G′ mutation alone is functional. Bar chart of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 3. **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
Similar articles
- The dyslexia-associated protein KIAA0319 interacts with adaptor protein 2 and follows the classical clathrin-mediated endocytosis pathway.
Levecque C, Velayos-Baeza A, Holloway ZG, Monaco AP. Levecque C, et al. Am J Physiol Cell Physiol. 2009 Jul;297(1):C160-8. doi: 10.1152/ajpcell.00630.2008. Epub 2009 May 6. Am J Physiol Cell Physiol. 2009. PMID: 19419997 Free PMC article. - Clathrin functions in the absence of the terminal domain binding site for adaptor-associated clathrin-box motifs.
Collette JR, Chi RJ, Boettner DR, Fernandez-Golbano IM, Plemel R, Merz AJ, Geli MI, Traub LM, Lemmon SK. Collette JR, et al. Mol Biol Cell. 2009 Jul;20(14):3401-13. doi: 10.1091/mbc.e08-10-1082. Epub 2009 May 20. Mol Biol Cell. 2009. PMID: 19458198 Free PMC article. - AP-2-dependent internalization of potassium channel Kir2.3 is driven by a novel di-hydrophobic signal.
Mason AK, Jacobs BE, Welling PA. Mason AK, et al. J Biol Chem. 2008 Mar 7;283(10):5973-84. doi: 10.1074/jbc.M709756200. Epub 2008 Jan 7. J Biol Chem. 2008. PMID: 18180291 - Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection.
Traub LM. Traub LM. J Cell Biol. 2003 Oct 27;163(2):203-8. doi: 10.1083/jcb.200309175. J Cell Biol. 2003. PMID: 14581447 Free PMC article. Review. - Linking endocytic cargo to clathrin: structural and functional insights into coated vesicle formation.
Owen DJ. Owen DJ. Biochem Soc Trans. 2004 Feb;32(Pt 1):1-14. doi: 10.1042/bst0320001. Biochem Soc Trans. 2004. PMID: 14748702 Review.
Cited by
- Cellular and viral peptides bind multiple sites on the N-terminal domain of clathrin.
Muenzner J, Traub LM, Kelly BT, Graham SC. Muenzner J, et al. Traffic. 2017 Jan;18(1):44-57. doi: 10.1111/tra.12457. Epub 2016 Dec 14. Traffic. 2017. PMID: 27813245 Free PMC article. - An interaction between β'-COP and the ArfGAP, Glo3, maintains post-Golgi cargo recycling.
Xie B, Guillem C, Date SS, Cohen CI, Jung C, Kendall AK, Best JT, Graham TR, Jackson LP. Xie B, et al. J Cell Biol. 2023 Apr 3;222(4):e202008061. doi: 10.1083/jcb.202008061. Epub 2023 Feb 22. J Cell Biol. 2023. PMID: 36811888 Free PMC article. - Inhibition of endocytosis suppresses the nitric oxide-dependent release of Cl- in retinal amacrine cells.
Dunn VK, Gleason E. Dunn VK, et al. PLoS One. 2018 Jul 25;13(7):e0201184. doi: 10.1371/journal.pone.0201184. eCollection 2018. PLoS One. 2018. PMID: 30044876 Free PMC article. - Getting in touch with the clathrin terminal domain.
Lemmon SK, Traub LM. Lemmon SK, et al. Traffic. 2012 Apr;13(4):511-9. doi: 10.1111/j.1600-0854.2011.01321.x. Epub 2012 Jan 13. Traffic. 2012. PMID: 22239657 Free PMC article. Review. - Synthesis of the Pitstop family of clathrin inhibitors.
Robertson MJ, Deane FM, Stahlschmidt W, von Kleist L, Haucke V, Robinson PJ, McCluskey A. Robertson MJ, et al. Nat Protoc. 2014 Jul;9(7):1592-606. doi: 10.1038/nprot.2014.106. Epub 2014 Jun 12. Nat Protoc. 2014. PMID: 24922269
References
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. - PubMed
- Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10(9):583–596. - PubMed
- Unanue ER, Ungewickell E, Branton D. The binding of clathrin triskelions to membranes from coated vesicles. Cell. 1981;26(3 Pt 1):439–446. - PubMed
- Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404(1-2):173–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials