Calcium signaling around Mitochondria Associated Membranes (MAMs) - PubMed (original) (raw)
doi: 10.1186/1478-811X-9-19.
Jan M Suski, Chiara Agnoletto, Angela Bononi, Massimo Bonora, Elena De Marchi, Carlotta Giorgi, Saverio Marchi, Sonia Missiroli, Federica Poletti, Alessandro Rimessi, Jerzy Duszynski, Mariusz R Wieckowski, Paolo Pinton
Affiliations
- PMID: 21939514
- PMCID: PMC3198985
- DOI: 10.1186/1478-811X-9-19
Calcium signaling around Mitochondria Associated Membranes (MAMs)
Simone Patergnani et al. Cell Commun Signal. 2011.
Abstract
Calcium (Ca2+) homeostasis is fundamental for cell metabolism, proliferation, differentiation, and cell death. Elevation in intracellular Ca2+ concentration is dependent either on Ca2+ influx from the extracellular space through the plasma membrane, or on Ca2+ release from intracellular Ca2+ stores, such as the endoplasmic/sarcoplasmic reticulum (ER/SR). Mitochondria are also major components of calcium signalling, capable of modulating both the amplitude and the spatio-temporal patterns of Ca2+ signals. Recent studies revealed zones of close contact between the ER and mitochondria called MAMs (Mitochondria Associated Membranes) crucial for a correct communication between the two organelles, including the selective transmission of physiological and pathological Ca2+ signals from the ER to mitochondria. In this review, we summarize the most up-to-date findings on the modulation of intracellular Ca2+ release and Ca2+ uptake mechanisms. We also explore the tight interplay between ER- and mitochondria-mediated Ca2+ signalling, covering the structural and molecular properties of the zones of close contact between these two networks.
Figures
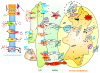
Figure 1
Representation of intracellular Ca2+ dynamics and MAMs proteins involved in ER-mitochondria Ca2+ cross-talk. A series of protein localized in MAMs (such as PML, AKT, grp-75, SIG-1R, Mfn-1/-2, BiP, AKT) regulate Ca2+ release from the ER and an efficient mitochondrial Ca2+ uptake, resulting in different functional outcomes. Cells generate Ca2+ signal through two mechanism that use internal and external sources of Ca2+. Calcium enters into the cell through channels and pumps situated on the plasma membrane; these are gated by voltage (VOCs) or external messengers (ROCs). A series of stimuli that act on cell surface receptors triggers the activation of PLC that catalyses the hydrolysis of phosphatidylinositol 4,5-biphosphate to IP3 and DAG. The binding of IP3 to its receptor IP3R stimulates ER Ca2+ release and consequently the transfer of Ca2+ (red dots) from ER to mitochondria. Mitochondrial surface directly interacts with the ER through contact sites defining hotspot Ca2+ signalling units. Mitochondrial Ca2+ import occurs through the mitochondrial Ca2+ uniporter (MCU) and the H+/Ca2+ exchanger LETM1; conversely, NCLX, mitochondrial Na+/Ca2+ exchanger, together with the PTP, export Ca2+ from the matrix. Ca2+ levels return to resting conditions through a series of channels and pumps: PMCA and NCX permit the ion extrusion into the extracellular milieu, SERCA (situated on the ER) and SPCA (on the Golgi apparatus) re-establish basal Ca2+ levels in intracellular stores. Abbreviations: ANT, adenosine nucleoside transporter; ETC, electron transport chain; HK, hexokinase; CD, cyclophilin D; CK, creatine kinase; BR, benzodiazepine receptor.
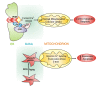
Figure 2
PML and p66Shc regulates cell span at the MAMs level. The tumor suppressor PML in resting conditions resides in a specific multi-protein complex with IP3R, PP2a and AKT, essential for a normal Ca2+ flux from ER to mitochondria and, consequently, for correct apoptosis levels (upper panel). Aging and ROS determine phosphorylation and accumulation of p66Shc in the MAMs fraction. The presence of phospho-p66Shc at the mitochondrial level determines alterations in mitochondrial homeostasis, including Ca2+ signalling, and ultimately increases apoptotic and senescence responses (lower panel).
Similar articles
- New functions of mitochondria associated membranes in cellular signaling.
van Vliet AR, Verfaillie T, Agostinis P. van Vliet AR, et al. Biochim Biophys Acta. 2014 Oct;1843(10):2253-62. doi: 10.1016/j.bbamcr.2014.03.009. Epub 2014 Mar 15. Biochim Biophys Acta. 2014. PMID: 24642268 Review. - The MAMs Structure and Its Role in Cell Death.
Wang N, Wang C, Zhao H, He Y, Lan B, Sun L, Gao Y. Wang N, et al. Cells. 2021 Mar 16;10(3):657. doi: 10.3390/cells10030657. Cells. 2021. PMID: 33809551 Free PMC article. Review. - A mathematical model of calcium dynamics: Obesity and mitochondria-associated ER membranes.
Han JM, Periwal V. Han JM, et al. PLoS Comput Biol. 2019 Aug 22;15(8):e1006661. doi: 10.1371/journal.pcbi.1006661. eCollection 2019 Aug. PLoS Comput Biol. 2019. PMID: 31437152 Free PMC article. - The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria.
Hajnóczky G, Csordás G, Madesh M, Pacher P. Hajnóczky G, et al. J Physiol. 2000 Nov 15;529 Pt 1(Pt 1):69-81. doi: 10.1111/j.1469-7793.2000.00069.x. J Physiol. 2000. PMID: 11080252 Free PMC article. Review. - Mitochondria-associated membranes (MAMs) as hotspot Ca(2+) signaling units.
Bononi A, Missiroli S, Poletti F, Suski JM, Agnoletto C, Bonora M, De Marchi E, Giorgi C, Marchi S, Patergnani S, Rimessi A, Wieckowski MR, Pinton P. Bononi A, et al. Adv Exp Med Biol. 2012;740:411-37. doi: 10.1007/978-94-007-2888-2_17. Adv Exp Med Biol. 2012. PMID: 22453952 Review.
Cited by
- STIM1 Deficiency Leads to Specific Down-Regulation of ITPR3 in SH-SY5Y Cells.
Pascual-Caro C, Orantos-Aguilera Y, Sanchez-Lopez I, de Juan-Sanz J, Parys JB, Area-Gomez E, Pozo-Guisado E, Martin-Romero FJ. Pascual-Caro C, et al. Int J Mol Sci. 2020 Sep 9;21(18):6598. doi: 10.3390/ijms21186598. Int J Mol Sci. 2020. PMID: 32916960 Free PMC article. - Disruption of mitochondria-associated ER membranes impairs insulin sensitivity and thermogenic function of adipocytes.
Wang CH, Wang CH, Hung PJ, Wei YH. Wang CH, et al. Front Cell Dev Biol. 2022 Sep 9;10:965523. doi: 10.3389/fcell.2022.965523. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158195 Free PMC article. - Impaired mitochondrial function in psychiatric disorders.
Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Manji H, et al. Nat Rev Neurosci. 2012 Apr 18;13(5):293-307. doi: 10.1038/nrn3229. Nat Rev Neurosci. 2012. PMID: 22510887 Review. - Loss of Phagocytic and Antigen Cross-Presenting Capacity in Aging Dendritic Cells Is Associated with Mitochondrial Dysfunction.
Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, Janssen EM. Chougnet CA, et al. J Immunol. 2015 Sep 15;195(6):2624-32. doi: 10.4049/jimmunol.1501006. Epub 2015 Aug 5. J Immunol. 2015. PMID: 26246142 Free PMC article. - Mitochondrial Ca2+ controls pancreatic cancer growth and metastasis by regulating epithelial cell plasticity.
Weissenrieder JS, Peura J, Paudel U, Bhalerao N, Weinmann N, Johnson C, Wengyn M, Drager R, Furth EE, Simin K, Ruscetti M, Stanger BZ, Rustgi AK, Pitarresi JR, Foskett JK. Weissenrieder JS, et al. bioRxiv [Preprint]. 2024 Aug 9:2024.08.08.607195. doi: 10.1101/2024.08.08.607195. bioRxiv. 2024. PMID: 39149344 Free PMC article. Preprint.
References
- Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. 2004;2004(215):re1. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous