Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera - PubMed (original) (raw)
Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera
Eve Draper et al. Vaccine. 2011.
Abstract
The majority of cervical cancers are associated with infection by one or more Human Papillomavirus (HPV) types from just two distinct Alpha-Papillomavirus species groups, A7 and A9. The extent to which the current HPV16/18 vaccines will protect against other genetically related HPV types is of interest to inform vaccine implementation, cervical disease surveillance and the development of second generation HPV vaccines. The aim of this study was to determine the frequency and titer of neutralizing antibodies against a range of A7 (18, 39, 45, 59, 68) and A9 (16, 31, 33, 35, 52, 58) HPV types using sera from individuals immunized with the bivalent HPV vaccine within the school-based, UK national HPV immunization programme. Serum samples were collected from 69 girls aged 13-14 years, a median 5.9 months (inter-quartile range, IQR, 5.7-6.0) after their third vaccine dose. Cross-neutralizing antibodies against HPV31, HPV33, HPV35 and HPV45 were common and strongly associated with the titer for the related vaccine-type, but were considerably lower (<1%) than their related vaccine type-specific response. The low prevalence of these HPV types in the population and the ages within the study cohort suggest these responses are due to vaccination. It is unclear whether such low levels of neutralizing antibodies would be sufficient to protect at the site of infection in the absence of other immune effectors but the coincidence with HPV types reported from efficacy studies is intriguing. The utility of neutralizing antibodies as surrogate markers of protection remains to be determined.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
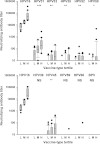
Fig. 1
Cross-neutralizing antibody titers are related to vaccine-type neutralizing antibody titers for A9 HPV types (top panel) and A7 HPV types (bottom panel). Neutralizing antibody data for non-vaccine types segregated according to Low (L), Middle (M) and High (H) vaccine-type tertiles. Plot shows box (median, IQR), whisker (±1.5 IQR) and outliers (>1.5 IQR). p values represent association by trend across tertiles: *p < 0.05; **_p_ < 0.01; ***_p_ < 0.001; NS_p_ > 0.05.
Similar articles
- Cross-neutralizing antibodies elicited by the Cervarix® human papillomavirus vaccine display a range of Alpha-9 inter-type specificities.
Bissett SL, Draper E, Myers RE, Godi A, Beddows S. Bissett SL, et al. Vaccine. 2014 Feb 26;32(10):1139-46. doi: 10.1016/j.vaccine.2014.01.008. Epub 2014 Jan 15. Vaccine. 2014. PMID: 24440205 Free PMC article. - Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes.
Barzon L, Squarzon L, Masiero S, Pacenti M, Marcati G, Mantelli B, Gabrielli L, Pascucci MG, Lazzarotto T, Caputo A, Palù G. Barzon L, et al. Vaccine. 2014 Sep 15;32(41):5357-62. doi: 10.1016/j.vaccine.2014.07.014. Epub 2014 Jul 18. Vaccine. 2014. PMID: 25045814 - Relationship between Humoral Immune Responses against HPV16, HPV18, HPV31 and HPV45 in 12-15 Year Old Girls Receiving Cervarix® or Gardasil® Vaccine.
Godi A, Bissett SL, Miller E, Beddows S. Godi A, et al. PLoS One. 2015 Oct 23;10(10):e0140926. doi: 10.1371/journal.pone.0140926. eCollection 2015. PLoS One. 2015. PMID: 26495976 Free PMC article. - Seropositivity to non-vaccine incorporated genotypes induced by the bivalent and quadrivalent HPV vaccines: A systematic review and meta-analysis.
Bissett SL, Godi A, Jit M, Beddows S. Bissett SL, et al. Vaccine. 2017 Jul 13;35(32):3922-3929. doi: 10.1016/j.vaccine.2017.06.028. Epub 2017 Jun 17. Vaccine. 2017. PMID: 28633892 Review. - Developments in L2-based human papillomavirus (HPV) vaccines.
Schellenbacher C, Roden RBS, Kirnbauer R. Schellenbacher C, et al. Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
Cited by
- Untangling the dynamics of persistence and colonization in microbial communities.
Ranjeva SL, Mihaljevic JR, Joseph MB, Giuliano AR, Dwyer G. Ranjeva SL, et al. ISME J. 2019 Dec;13(12):2998-3010. doi: 10.1038/s41396-019-0488-7. Epub 2019 Aug 23. ISME J. 2019. PMID: 31444482 Free PMC article. - Impact of smoking on the quantity and quality of antibodies induced by human papillomavirus type 16 and 18 AS04-adjuvanted virus-like-particle vaccine - a pilot study.
Namujju PB, Pajunen E, Simen-Kapeu A, Hedman L, Merikukka M, Surcel HM, Kirnbauer R, Apter D, Paavonen J, Hedman K, Lehtinen M. Namujju PB, et al. BMC Res Notes. 2014 Jul 11;7:445. doi: 10.1186/1756-0500-7-445. BMC Res Notes. 2014. PMID: 25011477 Free PMC article. - Naturally Occurring Major and Minor Capsid Protein Variants of Human Papillomavirus 45 (HPV45): Differential Recognition by Cross-Neutralizing Antibodies Generated by HPV Vaccines.
Godi A, Facchetti A, Bissett SL, Cocuzza C, Miller E, Beddows S. Godi A, et al. J Virol. 2015 Dec 30;90(6):3247-52. doi: 10.1128/JVI.02859-15. J Virol. 2015. PMID: 26719255 Free PMC article. - Human papillomavirus 16L1-58L2 chimeric virus-like particles elicit durable neutralizing antibody responses against a broad-spectrum of human papillomavirus types.
Chen X, Liu H, Wang Z, Wang S, Zhang T, Hu M, Qiao L, Xu X. Chen X, et al. Oncotarget. 2017 Jul 18;8(38):63333-63344. doi: 10.18632/oncotarget.19327. eCollection 2017 Sep 8. Oncotarget. 2017. PMID: 28968993 Free PMC article. - Global evaluation of lineage-specific human papillomavirus capsid antigenicity using antibodies elicited by natural infection.
Kamuyu G, Coelho da Silva F, Tenet V, Schussler J, Godi A, Herrero R, Porras C, Mirabello L, Schiller JT, Sierra MS, Kreimer AR, Clifford GM, Beddows S. Kamuyu G, et al. Nat Commun. 2024 Feb 21;15(1):1608. doi: 10.1038/s41467-024-45807-w. Nat Commun. 2024. PMID: 38383518 Free PMC article.
References
- Munoz N., Bosch F.X., de Sanjose S., Herrero R., Castellsague X., Shah K.V. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. - PubMed
- Li N., Franceschi S., Howell-Jones R., Snijders P.J., Clifford G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2010;128(4):927–935. - PubMed
- Paavonen J., Naud P., Salmeron J., Wheeler C.M., Chow S.N., Apter D. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. - PubMed
- Kjaer S.K., Sigurdsson K., Iversen O.E., Hernandez-Avila M., Wheeler C.M., Perez G. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009;2(10):868–878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials