Use of Clostridium perfringens Enterotoxin and the Enterotoxin Receptor-Binding Domain (C-CPE) for Cancer Treatment: Opportunities and Challenges - PubMed (original) (raw)
Use of Clostridium perfringens Enterotoxin and the Enterotoxin Receptor-Binding Domain (C-CPE) for Cancer Treatment: Opportunities and Challenges
Zhijian Gao et al. J Toxicol. 2012.
Abstract
Clostridium perfringens enterotoxin (CPE) causes the symptoms associated with several common gastrointestinal diseases. CPE is a 35 kDa polypeptide consisting of three structured domains, that is, C-terminal domain I (responsible for receptor binding), domain II (responsible for oligomerization and membrane insertion), and domain III (which may participate in physical changes when the CPE protein inserts into membranes). Native CPE binds to claudin receptors, which are components of the tight junction. The bound toxin then assembles into a hexameric prepore on the membrane surface, prior to the insertion of this oligomer into membranes to form an active pore. The toxin is especially lethal for cells expressing large amounts of claudin-3 or -4, which includes many cancer cells. Initial studies suggest that native CPE has potential usefulness for treating several cancers where claudin CPE receptors are overexpressed. However, some challenges with immunogenicity, toxicity, and (possibly) the development of resistance may need to be overcome. An alternative approach now being explored is to utilize C-CPE, which corresponds approximately to receptor binding domain I, to enhance paracellular permeability and delivery of chemotherapeutic agents against cancer cells. Alternatively, C-CPE fusion proteins may prove superior to use of native CPE for cancer treatment. Finally, C-CPE may have application for other medical treatments, including vaccination or increasing drug absorption. The coming years should witness increasing exploitation of this otherwise formidable toxin.
Figures
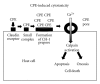
Figure 1
Model for CPE-induced cytotoxicity. CPE first binds to claudin receptors to form an ~90 kDa small complex. Six small complexes are then thought to oligomerize on the membrane surface to form a CH-1 prepore. The prepore then inserts into membranes to form the active pore. This results in entry of calcium into cells, which activates calpain. When a high CPE dose is used, there is substantial entry of calcium into cells, causing a strong calpain activation; this results in cell death by oncosis. Lower CPE doses cause more limited calcium influx and thus a milder calpain activation; these cells die by classical caspase 3-mediated apoptosis.
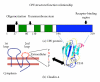
Figure 2
CPE structure versus function relationship. Panel A shows the functional regions of CPE, including the unstructured N-terminal sequences comprising amino acids 1–37 (black box), the domain II sequences mediating oligomerization and membrane insertion (white boxes), the domain III sequences that may participate when CPE inserts into membranes (green boxes), and the domain I sequences that mediate CPE receptor binding (blue box). Shown below the drawing is the structure of domain I (used with permission from [17]), including three tyrosine residues that interact with claudin receptors. Panel B shows the predicted structure of claudins. The amino acids in the turn region of extracellular loop 2 are shown to the right of claudin, with the two residues (N and, to a lesser extent, L) important for CPE binding highlighted in red.
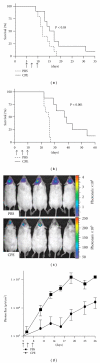
Figure 3
Efficacy of CPE in the treatment of breast cancer brain metastasis. (a, b) Brain tumors were established in mice using the human breast cancer cell line MDA-MB-468 and the murine breast cancer cell line NT2.5-luc. Tumors were treated by intracranial administration of 0.5 _μ_g CPE versus PBS on days 5, 7, and 9. (c, d) For the NT2.5-luc brain tumor model, noninvasive bioluminescent imaging was done twice per week beginning on day 4. Bioluminescent images from five representative mice are shown for each experimental group at day 19 (c). Photon flux was measured over the indicated time course as an indication of tumor growth (d). Differences in survival between experimental groups were analyzed using the log-rank test. Reproduced with permission from [20].
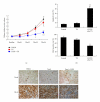
Figure 4
Combination therapy of C-CPE and taxol attenuates EOC xenograft growth in vivo. Female SCID mice were inoculated s.c. with 5 × 106 SKOV-3 cells. Four weeks later, the mice harboring large tumor burden were intraperitoneally administered with taxol alone (20 mg/kg), taxol combined with C-CPE (0.1 mg/kg), or vehicle (PBS) twice a week for 4 weeks. (a) Growth curves of tumors were presented as the mean volume normalized to the start volume. *The combination of C-CPE and taxol led to a significant tumor suppression compared with vehicle or taxol alone (P < 0.05). (b) After 4 weeks of treatment, immunostaining of Ki67 and TUNEL was performed to evaluate cell proliferation and apoptosis in EOC xenografts. *P < 0.05; **P < 0.001. Reproduced with permission from [35].
Similar articles
- The interaction of Clostridium perfringens enterotoxin with receptor claudins.
Shrestha A, Uzal FA, McClane BA. Shrestha A, et al. Anaerobe. 2016 Oct;41:18-26. doi: 10.1016/j.anaerobe.2016.04.011. Epub 2016 Apr 16. Anaerobe. 2016. PMID: 27090847 Free PMC article. Review. - Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications.
Freedman JC, Shrestha A, McClane BA. Freedman JC, et al. Toxins (Basel). 2016 Mar 16;8(3):73. doi: 10.3390/toxins8030073. Toxins (Basel). 2016. PMID: 26999202 Free PMC article. Review. - The complex interactions between Clostridium perfringens enterotoxin and epithelial tight junctions.
McClane BA. McClane BA. Toxicon. 2001 Nov;39(11):1781-91. doi: 10.1016/s0041-0101(01)00164-7. Toxicon. 2001. PMID: 11595640 Review. - Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects of Clostridium perfringens enterotoxin.
Shrestha A, McClane BA. Shrestha A, et al. mBio. 2013 Jan 15;4(1):e00594-12. doi: 10.1128/mBio.00594-12. mBio. 2013. PMID: 23322640 Free PMC article. - Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants.
Robertson SL, Smedley JG 3rd, Singh U, Chakrabarti G, Van Itallie CM, Anderson JM, McClane BA. Robertson SL, et al. Cell Microbiol. 2007 Nov;9(11):2734-55. doi: 10.1111/j.1462-5822.2007.00994.x. Epub 2007 Jun 24. Cell Microbiol. 2007. PMID: 17587331
Cited by
- Special Issue "Bacterial Toxins and Cancer".
Travaglione S, Carlini F, Maroccia Z, Fabbri A. Travaglione S, et al. Int J Mol Sci. 2024 Feb 9;25(4):2128. doi: 10.3390/ijms25042128. Int J Mol Sci. 2024. PMID: 38396805 Free PMC article. - Prevalence and clinical significance of Claudin-3 expression in cancer: a tissue microarray study on 14,966 tumor samples.
Büyücek S, Schraps N, Menz A, Lutz F, Chirico V, Viehweger F, Dum D, Schlichter R, Hinsch A, Fraune C, Bernreuther C, Kluth M, Hube-Magg C, Möller K, Reiswich V, Luebke AM, Lebok P, Weidemann S, Sauter G, Lennartz M, Jacobsen F, Clauditz TS, Marx AH, Simon R, Steurer S, Burandt E, Gorbokon N, Minner S, Krech T, Freytag M. Büyücek S, et al. Biomark Res. 2024 Dec 10;12(1):154. doi: 10.1186/s40364-024-00702-w. Biomark Res. 2024. PMID: 39658782 Free PMC article. - Claudin-3 expression increases the malignant potential of lung adenocarcinoma cells: role of epidermal growth factor receptor activation.
Zhang L, Wang Y, Zhang B, Zhang H, Zhou M, Wei M, Dong Q, Xu Y, Wang Z, Gao L, Qu Y, Shi B, Zhu J, Yin Y, Chen Y, Sun L, Zhang W, Xu S, Ying G, Wang C. Zhang L, et al. Oncotarget. 2017 Apr 4;8(14):23033-23047. doi: 10.18632/oncotarget.14974. Oncotarget. 2017. PMID: 28160565 Free PMC article. - Development of Human Monoclonal Antibody for Claudin-3 Overexpressing Carcinoma Targeting.
Yang H, Park H, Lee YJ, Choi JY, Kim T, Rajasekaran N, Lee S, Song K, Hong S, Choi JS, Shim H, Kim YD, Hwang S, Choi YL, Shin YK. Yang H, et al. Biomolecules. 2019 Dec 28;10(1):51. doi: 10.3390/biom10010051. Biomolecules. 2019. PMID: 31905631 Free PMC article. - Tight Junction Protein Signaling and Cancer Biology.
Nehme Z, Roehlen N, Dhawan P, Baumert TF. Nehme Z, et al. Cells. 2023 Jan 6;12(2):243. doi: 10.3390/cells12020243. Cells. 2023. PMID: 36672179 Free PMC article. Review.
References
- McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. The enterotoxic clostridia. In: Falkow S, Dworkin M, Rosenburg E, Schleifer H, Stackebrandt E, editors. The Prokaryotes. New York, NY, USA: Springer; 2006. pp. 688–752.
- McClane BA. Clostridium perfringens. In: Doyle MP, Beuchat LR, editors. Food Microbiology: Fundamentals and Frontiers. 3rd edition. Washington, DC, USA: ASM Press; 2007. pp. 423–444.
- Sarker MR, Carman RJ, McClane BA. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Molecular Microbiology. 1999;33(5):946–958. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous