Dendritic cell and macrophage heterogeneity in vivo - PubMed (original) (raw)
Dendritic cell and macrophage heterogeneity in vivo
Daigo Hashimoto et al. Immunity. 2011.
Abstract
Macrophage and dendritic cell (DC) are hematopoietic cells found in all tissues in the steady state that share the ability to sample the environment but have distinct function in tissue immunity. Controversies remain on the best way to distinguish macrophages from DCs in vivo. In this Perspective, we discuss how recent discoveries in the origin of the DC and macrophage lineage help establish key functional differences between tissue DC and macrophage subsets. We also emphasize the need to further understand the functional heterogeneity of the tissue DC and macrophage lineages to better comprehend the complex role of these cells in tissue homeostasis and immunity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
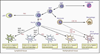
Figure 1. Origin of Batf3-IRF8-Id2-Dependent and -Independent Tissue-Resident DCs in Mice
Lymphoid and peripheral tissues have at least four distinct resident DC subsets: CD4− CD8α+, CD4+ CD8α−, CD103+ CD11b−, and CD103− CD11b+ DCs. A CD103+ CD11b+ DC subset has been best characterized within the small intestine. This figure illustrates the precursors, transcription factors (black), and cytokine receptors (red) required for the development of each population. Commitment to the mononuclear phagocyte lineage is determined at the stage of the macrophage-dendritic cell progenitor (MDP). Granulocyte/macrophage progenitor (GMP) give rise to MDP, which has lost granulocyte potential and gives rise only to monocytes and DCs restricted precursors (common DC precursors [CDPs]). CDPs have lost monocyte-macrophage differentiation potential and give rise exclusively to plasmacytoid DCs (pDCs) and pre-DCs circulating precursors that migrate to lymphoid tissues where they differentiate into lymphoid tissue CD4−CD8α+ and CD4+CD8α DCs and to nonlymphoid tissue to give rise to CD103+CD11b− DCs and CD103+CD11b+ DCs. Flt3 controls myeloid precursors commitment to the DC lineage as well as the differentiation of mature DCs in tissue. The transcription factors Batf3 Id2 and IRF8 control the differentiation of lymphoid tissue CD8+ DC and nonlymphoid tissues CD103+CD11b− DC but do not control CD11b+ DC differentiation in lymphoid and nonlymphoid tissues.
Similar articles
- Regulation of macrophage and dendritic cell responses by their lineage precursors.
Cortez-Retamozo V, Etzrodt M, Pittet MJ. Cortez-Retamozo V, et al. J Innate Immun. 2012;4(5-6):411-23. doi: 10.1159/000335733. Epub 2012 Mar 16. J Innate Immun. 2012. PMID: 22433183 Free PMC article. Review. - Bipotent or Oligopotent? A macrophage and DC progenitor revisited.
Onai N, Ohteki T. Onai N, et al. Immunity. 2014 Jul 17;41(1):5-7. doi: 10.1016/j.immuni.2014.07.004. Immunity. 2014. PMID: 25035946 - Deciphering the transcriptional network of the dendritic cell lineage.
Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M; Immunological Genome Consortium. Miller JC, et al. Nat Immunol. 2012 Sep;13(9):888-99. doi: 10.1038/ni.2370. Epub 2012 Jul 15. Nat Immunol. 2012. PMID: 22797772 Free PMC article. - Lymphoid tissue and plasmacytoid dendritic cells and macrophages do not share a common macrophage-dendritic cell-restricted progenitor.
Sathe P, Metcalf D, Vremec D, Naik SH, Langdon WY, Huntington ND, Wu L, Shortman K. Sathe P, et al. Immunity. 2014 Jul 17;41(1):104-15. doi: 10.1016/j.immuni.2014.05.020. Immunity. 2014. PMID: 25035955 - Human dendritic cell subsets.
Collin M, McGovern N, Haniffa M. Collin M, et al. Immunology. 2013 Sep;140(1):22-30. doi: 10.1111/imm.12117. Immunology. 2013. PMID: 23621371 Free PMC article. Review.
Cited by
- Physiopathological Roles of White Adiposity and Gut Functions in Neuroinflammation.
Spinedi E, Docena GH. Spinedi E, et al. Int J Mol Sci. 2024 Oct 31;25(21):11741. doi: 10.3390/ijms252111741. Int J Mol Sci. 2024. PMID: 39519291 Free PMC article. Review. - Message Transmission Between Adipocyte and Macrophage in Obesity.
Engin AB. Engin AB. Adv Exp Med Biol. 2024;1460:273-295. doi: 10.1007/978-3-031-63657-8_9. Adv Exp Med Biol. 2024. PMID: 39287855 Review. - Targeting Catechol-O-Methyltransferase Induces Mitochondrial Dysfunction and Enhances the Efficacy of Radiotherapy in Glioma.
Jiao M, Pirozzi CJ, Yu C, Bao X, Hu M, Pan D, Littleton S, Reynolds N, Saban DR, Li F, Li CY. Jiao M, et al. Cancer Res. 2024 Nov 4;84(21):3640-3656. doi: 10.1158/0008-5472.CAN-24-0134. Cancer Res. 2024. PMID: 39088832 Free PMC article. - Depleting profibrotic macrophages using bioactivated in vivo assembly peptides ameliorates kidney fibrosis.
Ouyang Q, Wang C, Sang T, Tong Y, Zhang J, Chen Y, Wang X, Wu L, Wang X, Liu R, Chen P, Liu J, Shen W, Feng Z, Zhang L, Sun X, Cai G, Li LL, Chen X. Ouyang Q, et al. Cell Mol Immunol. 2024 Aug;21(8):826-841. doi: 10.1038/s41423-024-01190-6. Epub 2024 Jun 13. Cell Mol Immunol. 2024. PMID: 38871810 - Detection of nitric oxide-mediated metabolic effects using real-time extracellular flux analysis.
Vagher B, Amiel E. Vagher B, et al. PLoS One. 2024 Mar 7;19(3):e0299294. doi: 10.1371/journal.pone.0299294. eCollection 2024. PLoS One. 2024. PMID: 38451983 Free PMC article.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. - PubMed
- Aliberti J, Schulz O, Pennington DJ, Tsujimura H, Reis e Sousa C, Ozato K, Sher A. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 2003;101:305–310. - PubMed
- Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. - PubMed
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI080884/AI/NIAID NIH HHS/United States
- R01 CA154947/CA/NCI NIH HHS/United States
- R01 HL086899/HL/NHLBI NIH HHS/United States
- HL086899/HL/NHLBI NIH HHS/United States
- AI095611/AI/NIAID NIH HHS/United States
- CA154947/CA/NCI NIH HHS/United States
- U01 AI095611/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources