Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis - PubMed (original) (raw)
. 2011 Sep 25;17(10):1242-50.
doi: 10.1038/nm.2491.
Martin Flamant, Sandra Schordan, Cécile Fligny, Elisabeth Rumpel, Marine Milon, Eric Schordan, Nathalie Sabaa, Sophie Vandermeersch, Ariane Galaup, Anita Rodenas, Ibrahim Casal, Susan W Sunnarborg, David J Salant, Jeffrey B Kopp, David W Threadgill, Susan E Quaggin, Jean-Claude Dussaule, Stéphane Germain, Laurent Mesnard, Karlhans Endlich, Claude Boucheix, Xavier Belenfant, Patrice Callard, Nicole Endlich, Pierre-Louis Tharaux
Affiliations
- PMID: 21946538
- PMCID: PMC3198052
- DOI: 10.1038/nm.2491
Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis
Guillaume Bollée et al. Nat Med. 2011.
Erratum in
- Nat Med. 2011 Nov;17(11):1521
- Nat Med. 2011 Oct;17(10):2 p following 1250
Abstract
Rapidly progressive glomerulonephritis (RPGN) is a life-threatening clinical syndrome and a morphological manifestation of severe glomerular injury that is marked by a proliferative histological pattern ('crescents') with accumulation of T cells and macrophages and proliferation of intrinsic glomerular cells. We show de novo induction of heparin-binding epidermal growth factor-like growth factor (HB-EGF) in intrinsic glomerular epithelial cells (podocytes) from both mice and humans with RPGN. HB-EGF induction increases phosphorylation of the epidermal growth factor receptor (EGFR, also known as ErbB1) in mice with RPGN. In HB-EGF-deficient mice, EGFR activation in glomeruli is absent and the course of RPGN is improved. Autocrine HB-EGF induces a phenotypic switch in podocytes in vitro. Conditional deletion of the Egfr gene from podocytes of mice alleviates the severity of RPGN. Likewise, pharmacological blockade of EGFR also improves the course of RPGN, even when started 4 d after the induction of experimental RPGN. This suggests that targeting the HB-EGF-EGFR pathway could also be beneficial in treatment of human RPGN.
Figures
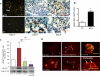
Figure 1. Induction of renal HB-EGF synthesis leads to glomerular activation of EGFR during RPGN
(a) Representative image of in situ hybridization study in NTS-injected wild-type (Hbegf (+/+)) animals, showing proHB-EGF expression in glomeruli (G), especially in parietal glomerular epithelial cells (Pec), in podocytes (day 4) and in crescents (Cr) (day 8). White arrow indicates abundant proHB-EGF mRNA expression in areas where tuft-capsular podocyte bridges were present. Scale bar (orange), 50 μm. (b) Quantification by real-time RT-PCR of proHB-EGF mRNA in freshly isolated podocytes on day 6 after nephrotoxic serum injection (NTS) and in podocytes from non-injected control mice (CT) (n=3 per group). * P<0.05 versus controls. (c) Western blot analysis of phosphorylated EGFR and total EGFR in the renal cortex from non-challenged controls (CT), wild-type (Hbegf (+/+)) mice infused with NTS ((+/+)NTS), HB-EGF deficient mice infused with NTS ((−/−)NTS), and from Hbegf (+/+) animals that were given intraperitoneal injections of the EGFR tyrosine kinase inhibitor AG1478 ((+/+)NTS+AG1478). Values reported are means ± sem. (n=6–8 per group). (d) Immunofluorescence staining for phosphoEGFR in renal cortex from controls (CT), NTS-injected Hbegf (+/+) mice ((+/+)NTS), HB-EGF deficient mice infused with NTS ((−/−)NTS), and from NTS-injected Hbegf (+/+) animals treated by AG1478 ((+/+)NTS+AG1478), on day 8 after NTS administration. * P<0.05 versus controls at baseline, ** P<0.01 versus controls at baseline. ## P<0.01 versus mice treated with vehicle only. Pec: parietal glomerular epithelial cells; G: glomerulus; Cr: crescent.
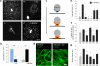
Figure 2. HB-EGF induces a migratory phenotype in podocytes in vitro
(a) Podocyte outgrowth over 6 days from decapsulated glomeruli of Hbegf (+/+) or Hbegf (−/−) mice (arrow). Cells are stained for WT-1 expression. (b) Outgrowth area from glomeruli of Hbegf (−/−) mice in the absence (light blue bar) or presence of the EGFR inhibitor AG1478 (500 nM) (AG, dark blue bar) and from glomeruli. Sparse outgrowth from glomeruli of Hbegf (−/−) mice in the absence (light grey bar) was rescued by addition of 30 nM HB-EGF (black bar). (c) Schematic drawing of podocyte outgrowth from isolated glomeruli, which was used as a combined migration/proliferation assay to assess the ability of crescent formation in vitro. Podocytes are in a stationary state (blue color) on the surface of capillary loops (grey circle), when glomeruli are plated. Subsequently, podocytes assume a migratory phenotye (orange color), characterized by apical protrusions, by attachment and by migration on the substratum. Later stages of outgrowth also involve proliferation. (d) Representative image of F-actin reorganisation and formation of ring-like actin structures (RiLiS) induced by HB-EGF (30 nM for 7 min) in differentiated podocytes, in the absence or presence of AG1478 (500 nM). The effect of HB-EGF to induce apical protrusions is abrogated in the presence of AG1478 (500 nM). (e) Quantitative analysis of RiLiS formation in differentiated podocytes. HB-EGF (30 nM) was added in the absence (Ctl) or presence of inhibitors: AG - AG1478 (500 nM), Wo - Wortmannin (100 nM), LY - LY294002 (30 μM), PD - PD98059 (25 μM), SB - SB203580 (25 μM). (f) BrdU incorporation in differentiated podocytes over 48 h. (g) Distance of migration of differentiated podocytes within 8 h in the wound assay. Data are means ± SEM (n=3–4 experiments). * P<0.05 vs. untreated Hbegf (+/+) glomeruli in (b) and vs. HB-EGF alone in (e–g). Scale bar : 300 μm in (a) and 30 μm in (d).
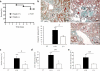
Figure 3. Deletion of Hbegf gene prevents fatal renal destruction
(a) Survival curve for challenged Hbegf (+/+) and Hbegf (−/−) mice. In all cases, death was associated with severe renal damage with macroscopic hematuria (blood leakage into the urine) and animals died from renal failure (with 100% crescentic and necrotizing lesions at autopsy). (b) Masson Trichrome staining of kidneys and proportion of crescentic glomeruli in control mice and in NTS-injected Hbegf (+/+) and Hbegf (−/−) mice (day 8 post NTS) (Cr: crescents, G: glomeruli, Tc: tubules with proteinaceous casts) Scale bar, 50μm. (c) Ascites score as an index of albumin plasma loss and water and sodium retention, (d) albuminuria and (e) blood urea nitrogen concentrations in NTS-challenged Hbegf (+/+) and Hbegf (−/−) animals on day 8 post NTS, and in unchallenged controls (CT). Values reported are means ± sem. (n=9–12 per group). * P<0.05 versus controls at baseline, ** P<0.01 versus baseline, *** P<0.001 versus baseline. # P<0.05 versus NTS-treated (+/+), ## P<0.01 versus NTS-treated (+/+).
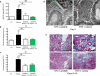
Figure 4. Selective deletion of Egfr from podocytes protects from RPGN
(a) Albuminuria (b) blood urea nitrogen concentration and (c) proportion of crescentic glomeruli in Pod-Tet on-Cre _Egfr_wt/wt and Pod-Tet on-Cre _Egfr_loxP/loxP mice, 8 days after NTS-induced RPGN (P<0.05 for all comparisons). (d) Survival curve of challenged Pod-Tet on-Cre _Egfr_loxP/loxP and littermate control mice in a severe model of RPGN. (* P<0.01). (e) Ultrastructural analysis of podocytes by transmission electron microscopy in NTS-treated Pod-Tet on-Cre _Egfr_wt/wt and Pod-Tet on-Cre _Egfr_loxP/loxP mice. More severe foot process effacement and irregular thickening of the GBM is visible in Pod-Tet on-Cre _Egfr_wt/wt animals. Scale bars: upper panels 2μm, lower panels 1μm.
Figure 5. Delayed EGFR tyrosine kinase inhibition stops the development of crescentic RPGN
(a) Quantification by western blot analysis of phosphorylated EGFR and total EGFR in the renal cortex from non-challenged controls (CT), NTS-injected mice treated with vehicle alone, and NTS-injected mice treated with erlotinib either started twelve hours before administration of NTS (days 0–14) or in a curative protocol, started four days later (d4–14). Mice were euthanised after 14 days of RPGN. (b) Blood urea nitrogen concentration and (c) proportion of crescentic glomeruli in CT in the different groups of mice as in (a). Data are means ± sem, (n=9 mice per group). ** P<0.01 versus controls at baseline (CT), *** P<0.001 versus CT, ## P<0.01 versus vehicle, ## P<0.001 versus vehicle. (d) Ultrastructural analysis of podocytes by transmission electron microscopy in erlotinib-treated and vehicle-treated mice, five days after injection of NTS. Scale bar 2 μm. (e) Masson trichrome staining of renal cortex from a mouse treated with erlotinib (day 4–14) (left panel) and a vehicle-treated mouse (right panel) on day 14. Ne: necrotic glomerular lesions, Cr: cellular crescents, Tc: tubular proteinaceous casts, Infilt: diffuse CD3-positive cell infiltrates (Infilt) seen in vehicle-treated mice. Scale bar 100 μm.
Figure 6. HB-EGF expression is induced in human crescentic glomerulonephritis
Representative images of immunostaining for HB-EGF using monoclonal sc-74526 antibody in sections of kidney biopsies from 8 random subjects diagnosed with non crescentic glomerulopathies (upper panels), including diabetic nephropathy, amyloidosis, minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), IgA nephropathy (IgAN), and membranous nephropathy (MN). Similarly, lower panels show immunostaining for HB-EGF in renal biopsies from 8 random subjects RPGN of various etiologies, including lupus nephritis, microscopic polyangiitis (MP), endocarditis (End), Goodpasture disease (Gp), and Wegener disease (Wg). Scale bar 50 μm.
Comment in
- EGFR signaling in podocytes at the root of glomerular disease.
Harris R. Harris R. Nat Med. 2011 Oct 11;17(10):1188-9. doi: 10.1038/nm.2455. Nat Med. 2011. PMID: 21988993 Free PMC article.
Similar articles
- EGFR signaling in podocytes at the root of glomerular disease.
Harris R. Harris R. Nat Med. 2011 Oct 11;17(10):1188-9. doi: 10.1038/nm.2455. Nat Med. 2011. PMID: 21988993 Free PMC article. - Epidermal growth factor receptor mediates injury in rapidly progressive glomerular disease.
Brandt S, Mertens PR. Brandt S, et al. Int Urol Nephrol. 2012 Jun;44(3):971-5. doi: 10.1007/s11255-012-0159-3. Epub 2012 Mar 24. Int Urol Nephrol. 2012. PMID: 22447112 Review. No abstract available. - Epidermal growth factor: a new therapeutic target in glomerular disease.
Flamant M, Bollée G, Hénique C, Tharaux PL. Flamant M, et al. Nephrol Dial Transplant. 2012 Apr;27(4):1297-304. doi: 10.1093/ndt/gfs030. Nephrol Dial Transplant. 2012. PMID: 22467748 Review. - Transactivation of the epidermal growth factor receptor by angiotensin II in glomerular podocytes.
Flannery PJ, Spurney RF. Flannery PJ, et al. Nephron Exp Nephrol. 2006;103(3):e109-18. doi: 10.1159/000092196. Epub 2006 Mar 22. Nephron Exp Nephrol. 2006. PMID: 16554661 - EGFR is dispensable for c-Met-mediated proliferation and survival activities in mouse adult liver oval cells.
Martínez-Palacián A, Del Castillo G, Herrera B, Fernández M, Roncero C, Fabregat I, Sánchez A. Martínez-Palacián A, et al. Cell Signal. 2012 Feb;24(2):505-513. doi: 10.1016/j.cellsig.2011.09.031. Epub 2011 Oct 7. Cell Signal. 2012. PMID: 22001397
Cited by
- Targeting the epidermal growth factor receptor (EGFR/ErbB) for the potential treatment of renal pathologies.
Tawengi M, Al-Dali Y, Tawengi A, Benter IF, Akhtar S. Tawengi M, et al. Front Pharmacol. 2024 Aug 21;15:1394997. doi: 10.3389/fphar.2024.1394997. eCollection 2024. Front Pharmacol. 2024. PMID: 39234105 Free PMC article. Review. - EGFR-ErbB2 dual kinase inhibitor lapatinib decreases autoantibody levels and worsens renal disease in Interferon α-accelerated murine lupus.
Gallo PM, Chain RW, Xu J, Whiteman LM, Palladino A, Caricchio R, Costa-Reis P, Sullivan KE, Gallucci S. Gallo PM, et al. Int Immunopharmacol. 2024 Oct 25;140:112692. doi: 10.1016/j.intimp.2024.112692. Epub 2024 Jul 29. Int Immunopharmacol. 2024. PMID: 39079344 Free PMC article. - Peripheral blood cells RNA-seq identifies differentially expressed gene network linked to lymphocyte subsets alterations and active lupus nephritis associated with declines in renal function.
Chen YC, Yu HH, Hu YC, Yang YH, Lin YT, Wang LC, Chiang BL, Lee JH. Chen YC, et al. Heliyon. 2024 Jun 2;10(11):e32303. doi: 10.1016/j.heliyon.2024.e32303. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38912505 Free PMC article. - Cytoplasmic retention of the DNA/RNA-binding protein FUS ameliorates organ fibrosis in mice.
Chiusa M, Lee YA, Zhang MZ, Harris RC, Sherrill T, Lindner V, Brooks CR, Yu G, Fogo AB, Flynn CR, Zienkiewicz J, Hawiger J, Zent R, Pozzi A. Chiusa M, et al. J Clin Invest. 2024 Mar 15;134(6):e175158. doi: 10.1172/JCI175158. J Clin Invest. 2024. PMID: 38488009 Free PMC article. - Construction of a prognosis model of head and neck squamous cell carcinoma pyroptosis and an analysis of immuno-phenotyping based on bioinformatics.
Pan W, Huang W, Zheng J, Meng Z, Pan X. Pan W, et al. Transl Cancer Res. 2024 Jan 31;13(1):299-316. doi: 10.21037/tcr-23-922. Epub 2024 Jan 29. Transl Cancer Res. 2024. PMID: 38410218 Free PMC article.
References
- Jennette JC, Thomas DB. Crescentic glomerulonephritis. Nephrol Dial Transplant. 2001;16(Suppl 6):80–82. - PubMed
- Yoshizumi M, et al. Tumor necrosis factor increases transcription of the heparin-binding epidermal growth factor-like growth factor gene in vascular endothelial cells. J Biol Chem. 1992;267:9467–9469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK030932/DK/NIDDK NIH HHS/United States
- R01 CA043793-19/CA/NCI NIH HHS/United States
- CA43793/CA/NCI NIH HHS/United States
- R01 CA043793/CA/NCI NIH HHS/United States
- R56 DK030932/DK/NIDDK NIH HHS/United States
- R01 DK030932-17/DK/NIDDK NIH HHS/United States
- DK30932/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous