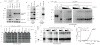STING is a direct innate immune sensor of cyclic di-GMP - PubMed (original) (raw)
STING is a direct innate immune sensor of cyclic di-GMP
Dara L Burdette et al. Nature. 2011.
Abstract
The innate immune system detects infection by using germline-encoded receptors that are specific for conserved microbial molecules. The recognition of microbial ligands leads to the production of cytokines, such as type I interferons (IFNs), that are essential for successful pathogen elimination. Cytosolic detection of pathogen-derived DNA is one major mechanism of inducing IFN production, and this process requires signalling through TANK binding kinase 1 (TBK1) and its downstream transcription factor, IFN-regulatory factor 3 (IRF3). In addition, a transmembrane protein called STING (stimulator of IFN genes; also known as MITA, ERIS, MPYS and TMEM173) functions as an essential signalling adaptor, linking the cytosolic detection of DNA to the TBK1-IRF3 signalling axis. Recently, unique nucleic acids called cyclic dinucleotides, which function as conserved signalling molecules in bacteria, have also been shown to induce a STING-dependent type I IFN response. However, a mammalian sensor of cyclic dinucleotides has not been identified. Here we report evidence that STING itself is an innate immune sensor of cyclic dinucleotides. We demonstrate that STING binds directly to radiolabelled cyclic diguanylate monophosphate (c-di-GMP), and we show that unlabelled cyclic dinucleotides, but not other nucleotides or nucleic acids, compete with c-di-GMP for binding to STING. Furthermore, we identify mutations in STING that selectively affect the response to cyclic dinucleotides without affecting the response to DNA. Thus, STING seems to function as a direct sensor of cyclic dinucleotides, in addition to its established role as a signalling adaptor in the IFN response to cytosolic DNA. Cyclic dinucleotides have shown promise as novel vaccine adjuvants and immunotherapeutics, and our results provide insight into the mechanism by which cyclic dinucleotides are sensed by the innate immune system.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
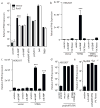
Figure 1. STING is sufficient to restore responsiveness to cyclic dinucleotides
a, Immortalized Myd88 −/−Trif −/− macrophages were transduced with retrovirus expressing RocR and stimulated for 6h. SeV, Sendai Virus; TMEV, Theiler’s Virus. IFN induction was measured by qRT-PCR and normalized to ribosomal protein 17 (Rps17). (b–d) HEK293T cells were transfected as indicated along with an IFN-luciferase reporter and luciferase activity was measured 6h following stimulation. VV 70-mer is a stimulatory dsDNA oligo derived from Vaccinia virus. ***, P < 0.001 e, Myd88 −/−Trif −/_−_macrophages were stimulated for 6h and IFN induction was measured as in a. Data are mean ± s.d. (n = 3) and are representative of at least three independent experiments.
Figure 2. STING binds cyclic dinucleotides
a, HEK293T cells were transfected as indicated and cell lysates were subjected to an in-vitro UV-crosslinking assay with c-di-GMP32. Samples were separated by SDS-PAGE and visualized by autoradiography or western blotting. b, HEK293T cells were transfected as in a and anti-HA immunoprecipitates were subjected to the c-di-GMP32 binding assay or stained with Colloidal Blue. c and d, HEK293T cells were transfected as in a and cell lysates were UV-crosslinked to c-di-GMP32 or GTP32 in the presence of cold competing nucleotides in 10-fold serial dilutions beginning at 1 mM, except for guanosine (0.1 mM), VV 70mer (500 μg/mL), poly(dAT:dTA) (50 μg/mL) and poly(I:C) (50 μg/mL). Arrow, STING; Asterisk, nonspecific bands. e, 1μg His6-mSTING 138–378 was separated by SDS-PAGE and stained with coomassie. f, His6-mSTING was analyzed as in c. g, C-di-GMP binding to purified His6-mSTING (10μM) was measured by equilibrium dialysis. Data are representative of three independent experiments.
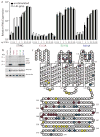
Figure 3. Mutational analysis of STING
a, HEK293T cells were transfected as indicated along with an IFN-luciferase reporter and luciferase activity was measured 6h following stimulation. b, HEK293T cells were transfected as in a except the lysates were subjected to the c-di-GMP32 binding assay as in Fig. 2a. c, Organization of STING based on the membrane topology prediction programs SOSUI, TMHMM, HMMTOP and TMPRED. Strongly predicted transmembrane domains are boxed; weakly predicted transmembrane domains have dashed boxes. Colored residues indicate mutant classes (see text): Class I (red), Class II (purple), Class III (green), Class IV (blue) and Class V (yellow). Bracketed mutations were made in combination. Data are representative of at least three independent experiments. Data are mean ± s.d. (n = 3).
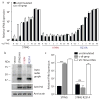
Figure 4. The IFN response to DNA and c-di-GMP can be uncoupled
a, HEK293T cells were transfected and IFN reporter activity was measured 6h following stimulation. b, HEK293T cells were transfected as in a except the lysates were subjected to the c-di-GMP32 binding assay as in Fig. 2a. c, Bone marrow-derived macrophages from _Sting_-deficient goldenticket mice were transduced with the indicated constructs. IFN induction by transfected cyclic-di-GMP and VV 70-mer dsDNA was measured by qRT-PCR and normalized to ribosomal protein 17 (Rps17). ***, P < 0.001. ns, not significant, P = 0.1205) Data are representative of at least three independent experiments. Data are mean± s.d. (n = 3).
Similar articles
- The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides.
Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. Sauer JD, et al. Infect Immun. 2011 Feb;79(2):688-94. doi: 10.1128/IAI.00999-10. Epub 2010 Nov 22. Infect Immun. 2011. PMID: 21098106 Free PMC article. - Crystallization studies of the murine c-di-GMP sensor protein STING.
Su YC, Tu ZL, Yang CY, Chin KH, Chuah ML, Liang ZX, Chou SH. Su YC, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Aug 1;68(Pt 8):906-10. doi: 10.1107/S1744309112024372. Epub 2012 Jul 31. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012. PMID: 22869119 Free PMC article. - Novel c-di-GMP recognition modes of the mouse innate immune adaptor protein STING.
Chin KH, Tu ZL, Su YC, Yu YJ, Chen HC, Lo YC, Chen CP, Barber GN, Chuah ML, Liang ZX, Chou SH. Chin KH, et al. Acta Crystallogr D Biol Crystallogr. 2013 Mar;69(Pt 3):352-66. doi: 10.1107/S0907444912047269. Epub 2013 Feb 16. Acta Crystallogr D Biol Crystallogr. 2013. PMID: 23519410 - Bacterial Cyclic Dinucleotides and the cGAS-cGAMP-STING Pathway: A Role in Periodontitis?
Elmanfi S, Yilmaz M, Ong WWS, Yeboah KS, Sintim HO, Gürsoy M, Könönen E, Gürsoy UK. Elmanfi S, et al. Pathogens. 2021 May 30;10(6):675. doi: 10.3390/pathogens10060675. Pathogens. 2021. PMID: 34070809 Free PMC article. Review. - The role of cGAS-STING signalling in liver diseases.
Chen R, Du J, Zhu H, Ling Q. Chen R, et al. JHEP Rep. 2021 Jun 24;3(5):100324. doi: 10.1016/j.jhepr.2021.100324. eCollection 2021 Oct. JHEP Rep. 2021. PMID: 34381984 Free PMC article. Review.
Cited by
- Structural mechanism of cytosolic DNA sensing by cGAS.
Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, Hornung V, Hopfner KP. Civril F, et al. Nature. 2013 Jun 20;498(7454):332-7. doi: 10.1038/nature12305. Epub 2013 May 30. Nature. 2013. PMID: 23722159 Free PMC article. - Hunting down the elusive cytosolic-DNA sensor.
Mathis D. Mathis D. Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2415648121. doi: 10.1073/pnas.2415648121. Epub 2024 Sep 19. Proc Natl Acad Sci U S A. 2024. PMID: 39297679 Free PMC article. - STING inhibitors target the cyclic dinucleotide binding pocket.
Hong Z, Mei J, Li C, Bai G, Maimaiti M, Hu H, Yu W, Sun L, Zhang L, Cheng D, Liao Y, Li S, You Y, Sun H, Huang J, Liu X, Lieberman J, Wang C. Hong Z, et al. Proc Natl Acad Sci U S A. 2021 Jun 15;118(24):e2105465118. doi: 10.1073/pnas.2105465118. Proc Natl Acad Sci U S A. 2021. PMID: 34099558 Free PMC article. - Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants.
Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ. Hanson MC, et al. J Clin Invest. 2015 Jun;125(6):2532-46. doi: 10.1172/JCI79915. Epub 2015 May 4. J Clin Invest. 2015. PMID: 25938786 Free PMC article. - At the Crossroads of the cGAS-cGAMP-STING Pathway and the DNA Damage Response: Implications for Cancer Progression and Treatment.
Korneenko TV, Pestov NB, Nevzorov IA, Daks AA, Trachuk KN, Solopova ON, Barlev NA. Korneenko TV, et al. Pharmaceuticals (Basel). 2023 Dec 1;16(12):1675. doi: 10.3390/ph16121675. Pharmaceuticals (Basel). 2023. PMID: 38139802 Free PMC article. Review.
References
- Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. - PubMed
- Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI080749-04/AI/NIAID NIH HHS/United States
- R37 AI075039/AI/NIAID NIH HHS/United States
- F32 AI091100/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- AI075039/AI/NIAID NIH HHS/United States
- AI063302/AI/NIAID NIH HHS/United States
- P01 AI063302/AI/NIAID NIH HHS/United States
- R01 AI075039-05/AI/NIAID NIH HHS/United States
- R01 AI080749/AI/NIAID NIH HHS/United States
- AI080749/AI/NIAID NIH HHS/United States
- R01 AI075039/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous