Digallate dimers of (-)-epigallocatechin gallate inactivate herpes simplex virus - PubMed (original) (raw)
Digallate dimers of (-)-epigallocatechin gallate inactivate herpes simplex virus
Charles E Isaacs et al. Antimicrob Agents Chemother. 2011 Dec.
Abstract
Topical microbicides are potentially an alternative method to vaccines for reducing the spread of herpes simplex virus (HSV). We have previously shown (S. Liu et al., Biochim. Biophys. Acta 1723:270-281, 2005) that the catechin (-)-epigallocatechin gallate (EGCG) inactivates HSV at neutral pH; however, to function in the female genital tract EGCG must also be effective at acidic pH. EGCG inactivated HSV-1 and HSV-2 at pH 8.0 by 3 log(10) to 4 log(10) but was ineffective at pH 5.7. The EGCG digallate dimers theasinensin A, P2, and theaflavin-3,3'-digallate (TF-3) inactivated both viruses by 3 log(10) to 4 log(10) at pH 5.7 and as much as 5 log(10) at pH 8.0. TF-3 inactivated HSV-1 and HSV-2 by 4 to 5 log(10) in the pH range of 4.0 to 5.7. Dimers with one gallate moiety had antiviral activity intermediate between the activities of EGCG and digallate dimers. Confocal and electron microscopy showed that theasinensin A did not damage Vero cells. All EGCG dimers inactivated enveloped viruses with class I, class II, and class III (HSV-1, HSV-2) fusion proteins more effectively than did monomeric EGCG. EGCG had no activity against the nonenveloped viruses tested, but TF-3 reduced the titer of 4 of 5 nonenveloped viruses by ≅2 to 3.5 log(10). Results also showed that HSV-1 glycoprotein B (gB) was aggregated more rapidly by theasinensin A than EGCG, which, when taken together with the nonenveloped virus data, suggests that dimers may inhibit the function of viral proteins required for infectivity. Digallate dimers of EGCG appear to have excellent potential as microbicidal agents against HSV at acidic and neutral pHs.
Figures
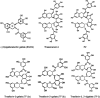
Fig. 1.
Structures of tea flavonoids with one gallate moiety (ring D), EGCG, TF-2a, and TF-2b, and two gallate moieties, theasinensin A, P2, and TF-3.
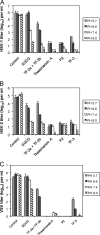
Fig. 2.
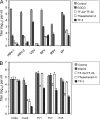
Effect of pH on the antiviral activity of monogallate and digallate tea flavonoids. Each compound was used at a concentration of 100 μM and incubated with virus from a clarified cell supernatant for 1 h at 37°C prior to titration with Vero cells. (A) HSV-1; (B) HSV-2; (C) VSV. Titers are the means ± standard deviations of results of three separate experiments.
Fig. 3.
Efficacy of digallate dimers in the pH range found vaginally. (A) HSV-1; (B) HSV-2. Each compound was used at a concentration of 100 μM. Isolates were prepared and titers were determined as described for Fig. 2.
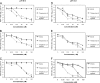
Fig. 4.
Effect of pH on the antiviral concentration range of monogallate and digallate tea flavonoids against HSV-1, pH 6.6 (A), HSV-2, pH 6.6 (B), VSV, pH 6.6 (C), HSV-1, pH 8.0 (D), HSV-2, pH 8.0 (E), and VSV, pH 8.0 (F). Isolates were prepared and titers were determined as described for Fig. 2.
Fig. 5.
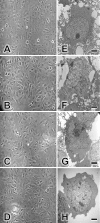
Effects of TF-3, theasinensin A, and EGCG on Vero cells. Vero cell monolayers were incubated for 1 h at 37°C in tissue culture medium with 1% FCS (A), medium with 100 μM EGCG (B), medium with 100 μM theasinensin A (C), or medium with 100 μM TF-3 (D). Monolayers were then examined by phase-contrast microscopy and photographed at ×20 magnification. Individual Vero cells were examined by EM as described in Materials and Methods. EGCG and dimers were utilized at 100 μM. (E) Control cells; (F) EGCG-treated cells; (G) theasinensin A-treated cells; (H) TF-3-treated cells. Scale bars are 2 μm (panels E to H).
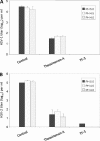
Fig. 6.
Inactivation of enveloped and nonenveloped viruses by EGCG and EGCG dimers. Each compound was used at a concentration of 100 μM and incubated for 1 h at pH 8.0. (A) Enveloped viruses: herpes simplex virus (HSV), vesicular stomatitis virus (VSV), Semliki Forest virus (SFV), respiratory syncytial virus (RSV), and measles virus (MV). (B) Nonenveloped viruses: coxsackie B4 virus (CVB4), coxsackie A9 virus (CVA9), poliovirus 1 (PV1), poliovirus 3 (PV3), and echovirus 6 (EV6). Viral titers are the means ± standard deviations of results of three separate experiments.
Fig. 7.
Effects of EGCG and theasinensin A on the formation of macromolecular complexes by HSV-1 envelope glycoproteins gB and gD. Theasinensin A or EGCG at a concentration of 550 μM was incubated with recombinant purified gB or gD for 1 or 24 h and then analyzed by SDS-PAGE, followed by Coomassie blue staining. The arrows indicate shifts in the banding pattern of gB following incubation with EGCG or theasinensin A.
Similar articles
- Theaflavin-3,3'-digallate and lactic acid combinations reduce herpes simplex virus infectivity.
Isaacs CE, Xu W. Isaacs CE, et al. Antimicrob Agents Chemother. 2013 Aug;57(8):3806-14. doi: 10.1128/AAC.00659-13. Epub 2013 May 28. Antimicrob Agents Chemother. 2013. PMID: 23716050 Free PMC article. - Antiviral Mechanism of Action of Epigallocatechin-3-_O_-gallate and Its Fatty Acid Esters.
Kaihatsu K, Yamabe M, Ebara Y. Kaihatsu K, et al. Molecules. 2018 Sep 27;23(10):2475. doi: 10.3390/molecules23102475. Molecules. 2018. PMID: 30262731 Free PMC article. Review. - Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus.
Isaacs CE, Wen GY, Xu W, Jia JH, Rohan L, Corbo C, Di Maggio V, Jenkins EC Jr, Hillier S. Isaacs CE, et al. Antimicrob Agents Chemother. 2008 Mar;52(3):962-70. doi: 10.1128/AAC.00825-07. Epub 2008 Jan 14. Antimicrob Agents Chemother. 2008. PMID: 18195068 Free PMC article. - Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate.
de Oliveira A, Adams SD, Lee LH, Murray SR, Hsu SD, Hammond JR, Dickinson D, Chen P, Chu TC. de Oliveira A, et al. Food Chem Toxicol. 2013 Feb;52:207-15. doi: 10.1016/j.fct.2012.11.006. Epub 2012 Nov 23. Food Chem Toxicol. 2013. PMID: 23182741 Free PMC article. - Antiviral activity of theaflavin digallate against herpes simplex virus type 1.
de Oliveira A, Prince D, Lo CY, Lee LH, Chu TC. de Oliveira A, et al. Antiviral Res. 2015 Jun;118:56-67. doi: 10.1016/j.antiviral.2015.03.009. Epub 2015 Mar 27. Antiviral Res. 2015. PMID: 25818500 Free PMC article.
Cited by
- Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses.
van de Sand L, Bormann M, Schmitz Y, Heilingloh CS, Witzke O, Krawczyk A. van de Sand L, et al. Viruses. 2021 Jul 16;13(7):1386. doi: 10.3390/v13071386. Viruses. 2021. PMID: 34372592 Free PMC article. Review. - Theaflavin-3,3'-digallate and lactic acid combinations reduce herpes simplex virus infectivity.
Isaacs CE, Xu W. Isaacs CE, et al. Antimicrob Agents Chemother. 2013 Aug;57(8):3806-14. doi: 10.1128/AAC.00659-13. Epub 2013 May 28. Antimicrob Agents Chemother. 2013. PMID: 23716050 Free PMC article. - Inhibitory effects of algal polysaccharide extract from Cladophora spp. against herpes simplex virus infection.
Srisai P, Suriyaprom S, Panya A, Pekkoh J, Tragoolpua Y. Srisai P, et al. Sci Rep. 2024 May 24;14(1):11914. doi: 10.1038/s41598-024-60941-7. Sci Rep. 2024. PMID: 38789457 Free PMC article. - Antimicrobial Activity of Chitosan-Based Films Enriched with Green Tea Extracts on Murine Norovirus, Escherichia coli, and Listeria innocua.
Amankwaah C, Li J, Lee J, Pascall MA. Amankwaah C, et al. Int J Food Sci. 2020 Jul 2;2020:3941924. doi: 10.1155/2020/3941924. eCollection 2020. Int J Food Sci. 2020. PMID: 32714970 Free PMC article. - Antiviral Mechanism of Action of Epigallocatechin-3-_O_-gallate and Its Fatty Acid Esters.
Kaihatsu K, Yamabe M, Ebara Y. Kaihatsu K, et al. Molecules. 2018 Sep 27;23(10):2475. doi: 10.3390/molecules23102475. Molecules. 2018. PMID: 30262731 Free PMC article. Review.
References
- Chu D.-C., Juneja L. R. 1997. General chemical composition of green tea and its infusion, p. 13–22.In Yamamoto T., Juneja L. R., Chu D.-C., Kim M. (ed.), Chemistry and applications of green tea. CRC Press, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous