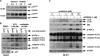Inhibition of mTOR kinase by AZD8055 can antagonize chemotherapy-induced cell death through autophagy induction and down-regulation of p62/sequestosome 1 - PubMed (original) (raw)
Inhibition of mTOR kinase by AZD8055 can antagonize chemotherapy-induced cell death through autophagy induction and down-regulation of p62/sequestosome 1
Shengbing Huang et al. J Biol Chem. 2011.
Abstract
AZD8055 is an ATP-competitive inhibitor of mammalian target of rapamycin (mTOR) that forms two multiprotein complexes, mTORC1 and mTORC2, and negatively regulates autophagy. We demonstrate that AZD8055 stimulates and potentiates chemotherapy-mediated autophagy, as shown by LC3I-II conversion and down-regulation of the ubiquitin-binding protein p62/sequestosome 1. AZD8055-induced autophagy was pro-survival as shown by its ability to attenuate cell death and DNA damage (p-H2AX), and to enhance clonogenic survival by cytotoxic chemotherapy. Autophagy inhibition by siRNA against Beclin 1 or LC3B, or by chloroquine, partially reversed the cytoprotective effect of AZD8055 that was independent of cell cycle inhibition. The pro-survival role of autophagy was confirmed using ectopic expression of Beclin 1 that conferred cytoprotection. To determine whether autophagy-mediated down-regulation of p62/sequestosome 1 contributes to its pro-survival role, we generated p62 knockdown cells using shRNA that showed protection from chemotherapy-induced cell death and DNA damage. We also overexpressed wild-type (wt) p62 that promoted chemotherapy-induced cell death, whereas mutated p62 at functional domains (PB1, UBA) failed to do so. The ability of ectopic wt p62 to promote cell death was blocked by AZD8055. AZD8055 was shown to inhibit phosphorylation of the autophagy-initiating kinase ULK1 at Ser(757) and inhibited known targets of mTORC1 (p-mTOR Ser(2448), p70S6K, p-S6, p4EBP1) and mTORC2 (p-mTOR Ser(2481), p-AKT Ser(473)). Knockdown of mTOR, but not Raptor or Rictor, reduced p-ULK1 at Ser(757) and enhanced chemotherapy-induced autophagy that resulted in a similar cytoprotective effect as shown for AZD8055. In conclusion, AZD8055 inhibits mTOR kinase and ULK1 phosphorylation to induce autophagy whose pro-survival effect is due, in part, to down-regulation of p62.
Figures
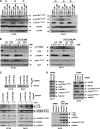
FIGURE 1.
AZD8055 treatment attenuates 5-FU-induced cell death in human colon cancer cell lines. A, AZD8055 (1 μ
m
) attenuates cell death induced by 5-FU (6 μ
m
: HT-29; 100 μ
m
: DLD1). Cells were incubated with study drugs for 3 days and annexin V/PI labeling was performed. Apoptosis is indicated by annexin V+ PI−-labeled cells and late apoptosis/necrosis is shown by annexin V+PI+ cells. Columns, mean of experiments conducted in triplicate; bars, S.D. B and C, AZD8055 attenuates caspase-9 and -3 cleavage (B) and DNA damage (C) induced by 5-FU (5 days) or CPT-11 (3 days) in HT-29 and/or DLD1 cells. DNA damage was measured by expression of p-H2AX that detects DNA double-stranded breaks. Tubulin served as a control for protein loading. D, suppression of colony formation by 5-FU is reduced by co-treatment with AZD8055 in both cell lines. Cells were incubated with drugs for 3 days and equal numbers of cells were replated and grown for 14 days in drug-free media. Colonies were visualized by staining with crystal violet.
FIGURE 2.
AZD8055-mediated cytoprotection is independent of cell cycle arrest. A, DLD1 and HT-29 cells were treated with rapamycin or AZD8055 at the indicated doses for 48 h and p27Kip1 expression levels were analyzed by immunoblotting. B, DLD1 cells were transduced with a lentivirus encoding GFP or p27Kip1. Cells were then treated with 5-FU or oxaliplatin for 5 days, and expression of p-H2Ax and cleaved caspase-9 and -3 proteins were measured by immunoblotting. C, AZD8055 enhances oxaliplatin-induced autophagy, as shown by LC3I-II conversion and reduced p62/SQSTM 1 expression, which is associated with attenuated DNA damage and caspase cleavage. DLD1 cells were treated with increasing doses of oxaliplatin alone or combined with AZD8055 for 5 days and expression of LC3, p62, p-H2Ax, and cleaved caspase-9 and -3 were analyzed by immunoblotting.
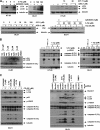
FIGURE 3.
AZD8055 induces autophagy to confer cytoprotection. A, AZD8055 induces autophagy and potentiates autophagy induced by 5-FU (5 d) or CPT-11 (3 days), as shown by conversion of LC3 I to II. Furthermore, treatment with the combination of AZD8055 and 5-FU (5 days) or CPT-11 (3 days) reduce p62/SQSTM 1 expression that is attenuated by the lysomotrophic autophagy inhibitor, chloroquine (CQ). B, DLD1 cells were treated with rapamycin (rap) or AZD8055 (AZD) for 3 days and LC3 and cleaved caspase-3 were measured by immunoblotting. HT-29 and DLD1 cells were treated with 5-FU alone or combined with rapamycin or AZD8055 at the indicated doses for 5 days. Expression of p-H2AX and caspase-9, -3 cleavage were detected using specific antibodies. C, autophagy inhibition by CQ accumulates LC3II and p62 and enhances caspase cleavage and DNA damage induced by 5-FU plus AZD8055 (5 days). Knockdown of Beclin 1 or LC3B using siRNA block LC3I-II conversion and enhance caspase cleavage and DNA damage induced by 5-FU combined with AZD8055 (5 days). *, nonspecific bands.
FIGURE 4.
Ectopic Beclin 1 expression attenuates 5-FU-induced apoptosis and DNA damage. A, HT-29 cells stably expressing a 3tag-Beclin 1 cDNA or an empty vector were treated with 5-FU in the presence or absence of CQ and protein expression was determined in whole cell lysates. B, clonogenic survival assay was performed using Beclin 1 expressing or control cells that were treated with 5-FU or DMSO for 3 days. Cells were then incubated in drug-free media for 7–14 days, and colonies were developed and stained with crystal violet, counted, and then normalized to vehicle (DMSO)-treated cells. Columns represent mean of experiments that were conducted in triplicate; bars, S.D. *, p < 0.05; **, p < 0.01.
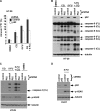
FIGURE 5.
Knockdown of p62/SQSTM 1 by shRNA is shown to attenuate 5-FU-induced apoptosis and DNA damage. A and B, p62 shRNA attenuates apoptosis induced by 5-FU (50 μ
m
) in the presence or absence of CQ, as shown by annexin V+ PI− labeling (36 h) [_A_] or caspase cleavage (B). Columns represent mean of triplicate experiments; bars, S.D. C and D, knockdown of p62 attenuates DNA damage (p-H2AX) induced by 5-FU alone or combined with CQ in HT-29 (C) and DLD-1 (D) cells.
FIGURE 6.
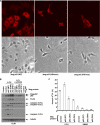
Ectopic expression of WT p62/SQSTM 1, but not functional p62 mutants, enhance 5-FU-induced apoptosis. A, mutations in p62 at UBA and PB1 domains were generated by site-directed mutagenesis and transduced into DLD1 cells. The staining pattern of ectopic 3 tandem tagged (3tag) p62 was then analyzed by immunofluoresence microscopy using an anti-FLAG antibody. In contrast to WT p62 cells that show punctate p62 staining, mutations at the UBA and PB1 domains resulted in loss of formation of p62 aggregates as shown by a diffuse staining pattern (top). Cellular morphology is shown by bright field images (bottom). B and C, ectopic expression of WT p62 is shown to enhance caspase cleavage (B) and apoptosis indicated by annexin V+PI− labeling (C) in cells treated with 5-FU (100 μ
m
). In contrast to WT p62, p62 mutations were shown to disrupt the ability of p62 to enhance apoptosis induction by 5-FU (B, C). Columns; mean of experiments conducted in triplicate; bars, S.D.
FIGURE 7.
AZD8055 inhibits ULK1 phosphorylation at Ser757 and blocks mTORC1/mTORC2 downstream signaling. A, cells were treated with rapamycin (rap) or AZD8055 (AZD) for the indicated doses and times, and p-mTOR (Ser2448 and Ser2481) and p-ULK1 (Ser757) were analyzed in whole cell lysates by immunoblotting. B, cells were treated with rapamycin or AZD8055 in the presence or absence of 5-FU for 24 h, and analysis of mTORC1 (p-P70S6K, p-S6, p-4E-BP1) and mTORC2 (p-AKT at Ser473) substrates was performed by immunoblotting. C, mTOR knockdown by shRNA is shown to potentiate 5-FU-induced autophagy, indicated by LC3I-II conversion that was associated with protection from 5-FU-induced DNA damage and apoptosis. Suppression of mTOR was achieved by transduction with two lentiviral shRNA sequences targeting mTOR (#1 or #2) compared with a scramble shRNA. Knockdown or control cells were treated with 5-FU (6 μ
m
for HT-29, 100 μ
m
for DLD1) for 5 days and LC3I-II conversion, DNA damage (p-H2AX) and caspase-9, -3 cleavage were probed. D, knockdown of mTOR, but not Raptor or Rictor, confers cytoprotection. DLD1 cells were transfected with mTOR, Raptor or Rictor siRNA for 3 days and phosphorylation of p-mTOR (Ser2448 and Ser2481), p-ULK1 (Ser757), as well as phosphorylation of the substrates of mTORC1 (p-S6, p-4EBP-1) or mTORC2 (p-AKT at Ser473) were analyzed. Raptor, Rictor or control knockdown cells were treated with 5-FU for 5 days, and expression of p-H2AX and caspase-9, -3 cleavage were measured.
Similar articles
- ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding.
Dunlop EA, Hunt DK, Acosta-Jaquez HA, Fingar DC, Tee AR. Dunlop EA, et al. Autophagy. 2011 Jul;7(7):737-47. doi: 10.4161/auto.7.7.15491. Epub 2011 Jul 1. Autophagy. 2011. PMID: 21460630 Free PMC article. - The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor activity in acute myeloid leukemia.
Willems L, Chapuis N, Puissant A, Maciel TT, Green AS, Jacque N, Vignon C, Park S, Guichard S, Herault O, Fricot A, Hermine O, Moura IC, Auberger P, Ifrah N, Dreyfus F, Bonnet D, Lacombe C, Mayeux P, Bouscary D, Tamburini J. Willems L, et al. Leukemia. 2012 Jun;26(6):1195-202. doi: 10.1038/leu.2011.339. Epub 2011 Dec 6. Leukemia. 2012. PMID: 22143671 - The association of AMPK with ULK1 regulates autophagy.
Lee JW, Park S, Takahashi Y, Wang HG. Lee JW, et al. PLoS One. 2010 Nov 3;5(11):e15394. doi: 10.1371/journal.pone.0015394. PLoS One. 2010. PMID: 21072212 Free PMC article. - LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.
Shaw RJ. Shaw RJ. Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review. - p62: a versatile multitasker takes on cancer.
Moscat J, Diaz-Meco MT. Moscat J, et al. Trends Biochem Sci. 2012 Jun;37(6):230-6. doi: 10.1016/j.tibs.2012.02.008. Epub 2012 Mar 15. Trends Biochem Sci. 2012. PMID: 22424619 Free PMC article. Review.
Cited by
- The role of stearoyl-coenzyme A desaturase 1 in clear cell renal cell carcinoma.
Wang H, Zhang Y, Lu Y, Song J, Huang M, Zhang J, Huang Y. Wang H, et al. Tumour Biol. 2016 Jan;37(1):479-89. doi: 10.1007/s13277-015-3451-x. Epub 2015 Jul 31. Tumour Biol. 2016. PMID: 26224474 - Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons.
Silva MC, Nandi GA, Tentarelli S, Gurrell IK, Jamier T, Lucente D, Dickerson BC, Brown DG, Brandon NJ, Haggarty SJ. Silva MC, et al. Nat Commun. 2020 Jun 26;11(1):3258. doi: 10.1038/s41467-020-16984-1. Nat Commun. 2020. PMID: 32591533 Free PMC article. - Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling.
Altomare DA, Khaled AR. Altomare DA, et al. Curr Med Chem. 2012;19(22):3748-62. doi: 10.2174/092986712801661130. Curr Med Chem. 2012. PMID: 22680924 Free PMC article. Review. - The combination of an mTORc1/TORc2 inhibitor with lapatinib is synergistic in bladder cancer in vitro.
Becker MN, Wu KJ, Marlow LA, Kreinest PA, Vonroemeling CA, Copland JA, Williams CR. Becker MN, et al. Urol Oncol. 2014 Apr;32(3):317-26. doi: 10.1016/j.urolonc.2013.06.002. Epub 2013 Sep 17. Urol Oncol. 2014. PMID: 24054871 Free PMC article. - Lower Beclin 1 downregulates HER2 expression to enhance tamoxifen sensitivity and predicts a favorable outcome for ER positive breast cancer.
Gu Y, Chen T, Li G, Xu C, Xu Z, Zhang J, He K, Zheng L, Guan Z, Su X, Cao J, Teng L. Gu Y, et al. Oncotarget. 2016 Aug 4;8(32):52156-52177. doi: 10.18632/oncotarget.11044. eCollection 2017 Aug 8. Oncotarget. 2016. PMID: 28881721 Free PMC article.
References
- Lindsley J. E., Rutter J. (2004) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 139, 543–559 - PubMed
- Guertin D. A., Sabatini D. M. (2007) Cancer Cell 12, 9–22 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K05 CA142885/CA/NCI NIH HHS/United States
- N01 CA015083/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- 1 K05 CA142885-01/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous