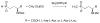In vitro phosphinate methylation by PhpK from Kitasatospora phosalacinea - PubMed (original) (raw)
. 2011 Oct 25;50(42):8986-8.
doi: 10.1021/bi201220r. Epub 2011 Sep 28.
Affiliations
- PMID: 21950770
- PMCID: PMC3214642
- DOI: 10.1021/bi201220r
In vitro phosphinate methylation by PhpK from Kitasatospora phosalacinea
Williard J Werner et al. Biochemistry. 2011.
Abstract
Radical S-adenosyl-L-methionine, cobalamin-dependent methyltransferases have been proposed to catalyze the methylations of unreactive carbon or phosphorus atoms in antibiotic biosynthetic pathways. To date, none of these enzymes has been purified or shown to be active in vitro. Here we demonstrate the activity of the P-methyltransferase enzyme, PhpK, from the phosalacine producer Kitasatospora phosalacinea. PhpK catalyzes the transfer of a methyl group from methylcobalamin to 2-acetylamino-4-hydroxyphosphinylbutanoate (N-acetyldemethylphosphinothricin) to form 2-acetylamino-4-hydroxymethylphosphinylbutanoate (N-acetylphosphinothricin). This transformation gives rise to the only carbon-phosphorus-carbon linkage known to occur in nature.
Figures
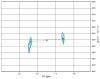
Figure 1
H-P gHSQC spectrum of the partially purified 13CH3-Cbl(III) PhpK reaction. The 13CH3 H-P crosspeaks are centered at (1.15, 43.1) ppm (designated by the “x”).
Scheme 1
Proposed P-methyl transfer reactions
Similar articles
- Efficient methylation of C2 in l-tryptophan by the cobalamin-dependent radical _S_-adenosylmethionine methylase TsrM requires an unmodified N1 amine.
Blaszczyk AJ, Wang B, Silakov A, Ho JV, Booker SJ. Blaszczyk AJ, et al. J Biol Chem. 2017 Sep 15;292(37):15456-15467. doi: 10.1074/jbc.M117.778548. Epub 2017 Jul 26. J Biol Chem. 2017. PMID: 28747433 Free PMC article. - Reaction Catalyzed by GenK, a Cobalamin-Dependent Radical S-Adenosyl-l-methionine Methyltransferase in the Biosynthetic Pathway of Gentamicin, Proceeds with Retention of Configuration.
Kim HJ, Liu YN, McCarty RM, Liu HW. Kim HJ, et al. J Am Chem Soc. 2017 Nov 15;139(45):16084-16087. doi: 10.1021/jacs.7b09890. Epub 2017 Nov 7. J Am Chem Soc. 2017. PMID: 29091410 Free PMC article. - GenK-catalyzed C-6' methylation in the biosynthesis of gentamicin: isolation and characterization of a cobalamin-dependent radical SAM enzyme.
Kim HJ, McCarty RM, Ogasawara Y, Liu YN, Mansoorabadi SO, LeVieux J, Liu HW. Kim HJ, et al. J Am Chem Soc. 2013 Jun 5;135(22):8093-6. doi: 10.1021/ja312641f. Epub 2013 May 21. J Am Chem Soc. 2013. PMID: 23679096 Free PMC article. - Radical-mediated enzymatic methylation: a tale of two SAMS.
Zhang Q, van der Donk WA, Liu W. Zhang Q, et al. Acc Chem Res. 2012 Apr 17;45(4):555-64. doi: 10.1021/ar200202c. Epub 2011 Nov 18. Acc Chem Res. 2012. PMID: 22097883 Free PMC article. Review. - Rapid and direct measurement of methyltransferase activity in about 30 min.
Hevel JM, Price OM. Hevel JM, et al. Methods. 2020 Mar 15;175:3-9. doi: 10.1016/j.ymeth.2019.10.002. Epub 2019 Oct 9. Methods. 2020. PMID: 31605745 Review.
Cited by
- Initial characterization of Fom3 from Streptomyces wedmorensis: The methyltransferase in fosfomycin biosynthesis.
Allen KD, Wang SC. Allen KD, et al. Arch Biochem Biophys. 2014 Feb 1;543:67-73. doi: 10.1016/j.abb.2013.12.004. Epub 2013 Dec 24. Arch Biochem Biophys. 2014. PMID: 24370735 Free PMC article. - TsrM as a Model for Purifying and Characterizing Cobalamin-Dependent Radical S-Adenosylmethionine Methylases.
Blaszczyk AJ, Wang RX, Booker SJ. Blaszczyk AJ, et al. Methods Enzymol. 2017;595:303-329. doi: 10.1016/bs.mie.2017.07.007. Epub 2017 Aug 21. Methods Enzymol. 2017. PMID: 28882204 Free PMC article. - Investigation of enzymatic C-P bond formation using multiple quantum HCP nuclear magnetic resonance spectroscopy.
Hu K, Werner WJ, Allen KD, Wang SC. Hu K, et al. Magn Reson Chem. 2015 Apr;53(4):267-72. doi: 10.1002/mrc.4190. Epub 2015 Jan 16. Magn Reson Chem. 2015. PMID: 25594737 Free PMC article. - Phosphonate biosynthesis and catabolism: a treasure trove of unusual enzymology.
Peck SC, van der Donk WA. Peck SC, et al. Curr Opin Chem Biol. 2013 Aug;17(4):580-8. doi: 10.1016/j.cbpa.2013.06.018. Epub 2013 Jul 17. Curr Opin Chem Biol. 2013. PMID: 23870698 Free PMC article. Review. - Radical SAM-mediated methylation reactions.
Fujimori DG. Fujimori DG. Curr Opin Chem Biol. 2013 Aug;17(4):597-604. doi: 10.1016/j.cbpa.2013.05.032. Epub 2013 Jul 5. Curr Opin Chem Biol. 2013. PMID: 23835516 Free PMC article. Review.
References
- Collinsová M, Jirácek J. Curr Med Chem. 2000;7:629–647. - PubMed
- Thompson CJ, Seto H. Genetics and Biochemistry of Antibiotic Production. 1995. pp. 197–222.
- Hoerlein G. Rev Environ Contam Tox. 1994;138:73–145. - PubMed
- Bayer E, Gugel KH, Hägele K, Hagenmaier H, Jessipow S, König WA, Zähner H. Helv Chim Acta. 1972;55:224–239. - PubMed
- Ogawa Y, Tsuruoka T, Inouye S, Niida T. Sci Reports Meiji Seika Kaisha. 1973;13:42–48.
Publication types
MeSH terms
Substances
Grants and funding
- P41 RR002301/RR/NCRR NIH HHS/United States
- T32 GM008336/GM/NIGMS NIH HHS/United States
- P41 GM066326/GM/NIGMS NIH HHS/United States
- S10 RR012948/RR/NCRR NIH HHS/United States
- RR0631401/RR/NCRR NIH HHS/United States
- P41GM66326/GM/NIGMS NIH HHS/United States
- T32GM008336/GM/NIGMS NIH HHS/United States
- S10 RR002781/RR/NCRR NIH HHS/United States
- S10 RR008438-01/RR/NCRR NIH HHS/United States
- S10 RR012948-01/RR/NCRR NIH HHS/United States
- P41RR02301/RR/NCRR NIH HHS/United States
- T32 GM008336-23/GM/NIGMS NIH HHS/United States
- S10 RR008438/RR/NCRR NIH HHS/United States
- P41 RR002301-24/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases