The Cutoff protein regulates piRNA cluster expression and piRNA production in the Drosophila germline - PubMed (original) (raw)
The Cutoff protein regulates piRNA cluster expression and piRNA production in the Drosophila germline
Attilio Pane et al. EMBO J. 2011.
Abstract
In a broad range of organisms, Piwi-interacting RNAs (piRNAs) have emerged as core components of a surveillance system that protects the genome by silencing transposable and repetitive elements. A vast proportion of piRNAs is produced from discrete genomic loci, termed piRNA clusters, which are generally embedded in heterochromatic regions. The molecular mechanisms and the factors that govern their expression are largely unknown. Here, we show that Cutoff (Cuff), a Drosophila protein related to the yeast transcription termination factor Rai1, is essential for piRNA production in germline tissues. Cuff accumulates at centromeric/pericentromeric positions in germ-cell nuclei and strongly colocalizes with the major heterochromatic domains. Remarkably, we show that Cuff is enriched at the dual-strand piRNA cluster 1/42AB and is likely to be involved in regulation of transcript levels of similar loci dispersed in the genome. Consistent with this observation, Cuff physically interacts with the Heterochromatin Protein 1 (HP1) variant Rhino (Rhi). Our results unveil a link between Cuff activity, heterochromatin assembly and piRNA cluster expression, which is critical for stem-cell and germ-cell development in Drosophila.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
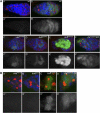
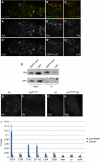
(A) The pGFP-ZenvAS reporter is deregulated in the germaria of cuff, aub, rhi and vas mutants. (A, A′) The pGFP-ZenvAS reporter is silenced in wild-type (wt) ovaries. (B, B′) A GFP sensor construct lacking the Zenv sequence is constitutively deregulated in wild-type ovaries. (C, C′) Expression of the pGFP-ZenvAS sensor transgene is visibly increased in stage 4-cell and 8-cell cysts of _vas_PH165 homozygous mutant germaria. (D, D′) Mutations in aub deregulate the pGFP-ZenvAS reporter in the late mitotic cyst. (E, E′) Expression of the reporter is dramatically upregulated in the cuff mutant germaria. (F, F′) Mutations in rhi cause a significant derepression of the sensor construct. The branching spectrosome/fusome, which interconnects the dividing cells in the mitotic cyst, is visualized with anti-α-Spectrin antibody (red). (A′–F′) GFP single channels. (B) The expression of the pGFP-ZenvAS sensor is differentially affected in stem cells of vas, aub, cuff and rhi mutants. The sensor construct is silenced in vas (A, A′) and aub (B, B′) mutant stem cells. Mutations in cuff (C, C′) and rhi (D, D′) cause a significant derepression of the reporter in stem-cell position. The spectrosome/fusome is visualized with anti-α-Spectrin antibody (red).
Figure 2
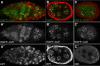
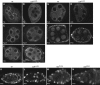
Cuff localizes to centromeric/pericentromeric and heterochromatic regions. (A–A″) Wild-type germarium expressing the EGFP–Cuff chimeric protein (A, green and A′, single channel) labelled with anti-HP1 antibody (A, red and A″, single channel). EGFP–Cuff localizes to nuclear speckles and appears enriched in heterochromatic regions marked with HP1 (arrows). (B–B″) EGFP–Cuff (B, green and B′, single channel) extensively overlaps with HP1 (B, red and B″, single channel) in nurse cell nuclei of stage 6 egg chambers (arrows). (C–C″) EGFP–Cuff (C, green and C′, single channel) ovaries labelled with anti-CID antibody (C, red and C″, single channel). Cuff is enriched in proximity of the centromeres (arrows).
Figure 3
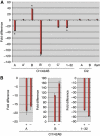
Cuff regulates piRNA cluster expression. (A) qRT–PCR analysis of the transcripts produced from cluster 1/42AB (regions A, A′, B, B′, C, C′ and 1–32) cluster 2 (regions A and B) and flam. Mutations in cuff cause a downregulation of the regions A, B′, C′ and 1–32 of cluster 1/42AB, while region B is upregulated. Regions A′ and C are not affected by mutations in cuff. Cluster 2 and flam appear unaffected in the cuff mutant. Asterisks mark the region of Cl1/42AB, which were subjected to strand-specific qRT–PCR (B). The fold difference between cuff mutant and wild type is reported on the Y axis. (B) Strand-specific qRT–PCR analysis of cluster 1/42AB regions A, B and 1–32. Mutations in cuff affect the expression levels of both plus and minus genomic DNA strands. The fold difference between cuff mutant and wild type is reported on the Y axis.
Figure 4
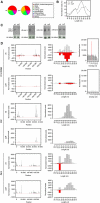
Analysis of the piRNA levels in the cuff mutant ovaries. (A) Pie chart displaying the total number of reads of small RNAs in cuff and control (wt) ovaries. (B) Length distribution of the uniquely mapped sequence reads in the cuff and control libraries. Mutations in cuff cause an approximate 74% reduction of the piRNA population. A second peak at 21/22 nt corresponds to a heterogeneous population formed by piRNAs and endo-siRNAs. This class of small RNAs is not dramatically affected in cuff ovaries. (C) Northern blot analyses on Het-A-specific and AT-chX-1 piRNAs in wild-type and cuff mutant ovaries. Both piRNAs are detected in the wild-type lane, while they are absent in the cuff lane. Northern blot analyses on the abundant miR-310 and miR-184 reveal that mutations in cuff do not affect the production/stability of these miRNAs. (D) Analysis of uniquely mapping piRNAs produced by cluster 1/42AB, cluster 2 and flam in wt (left) and cuff mutant (right). For cluster 1/42AB, piRNA densities along the cluster, length distribution and ping-pong signal are displayed. For cluster 2 and flam, piRNA densities along the cluster and length distribution are displayed.
Figure 5
Mutations in cuff strongly reduce the piRNA levels. Log2 ratio of the normalized number of piRNA reads between the cuff mutant and the wild type for the canonical transposon sequences (left). For each transposon, sense (+) and antisense (−) piRNAs are reported as reduced (green), unaffected (white) or increased (red); for the vast majority of the transposons, the corresponding piRNA set is reduced (colour key and histogram). The HeT-A, TART, rover and Zam transposable elements, which are analysed in more details (right), are highlighted (*). For each of these transposons, piRNA densities along the canonical sequence, length distribution and ping-pong signal are analysed in wt and cuff mutant ovaries. The blue line indicates the position of the Zam fragment adopted in the pGFP-ZenvAS reporter line (Desset et al, 2008).
Figure 6
Cuff and Rhi extensively colocalize in germline nuclear foci and physically interact. (A–A″) Immunostaining assay with anti-Cuff (A, red and A″, single channel) and anti-Rhi (A, green and A′, single channel) antibodies in wild-type germaria. (B–B″) Nurse cell nuclei of stage 10 egg chambers expressing EGFP–Cuff (B, green and B″, single channel) stained with anti-Rhi antibody (B, red and B′, single channel). (C–C″) Nurse cell nuclei of stage 10 egg chambers immunostained with anti-Cuff (C, red and C″, single channel) and ant-Rhi (C, green and C′, single channel) antibodies. Arrows indicate Cuff-positive foci, which do not colocalize with Rhi. DNA was labelled with Hoechst (A–C, blue). (D) Cuff and Rhi physically interact. IP on control lines (OrR) and EGFP–Cuff ovarian extracts was performed with anti-GFP antibodies and followed by western blotting with anti-Rhi antibodies. A 37-kDa band corresponding to the molecular weight of Rhi can be detected in the input extracts. Rhi is efficiently immunoprecipitated from EGFP–Cuff extracts, while a faint background signal is present in the OrR lane. (E–H) Immunostaining assays on stage 6–10 wt and cuff egg chambers. Rhi localizes to nuclear speckles in wt nurse cell nuclei (E), while it appears dispersed in the nucleoplasm of cuff egg chambers (F). In the reciprocal assay, Cuff localizes to nurse cell nuclear foci in wt stage 6–10 egg chambers (G), while it is dispersed in the nucleoplasm of rhi mutant ovaries. (I) Chromatin immunoprecipitation assay on ovarian extracts obtained from EGFP–Cuff expressing flies. Cuff is enriched in regions 1A, 1C and 1–32, but not in region 1B within cluster 1/42AB. Cuff does not display a significant enrichment at regions 2A and 2B within cluster 2 and in the flam locus. Primers for the rosy (ry) and rpr49 (rp49) genes were used as negative control. Blue bars represent the ChIP assay with anti-GFP antibody on EGFP–Cuff expressing ovaries, while red bars display the result of a control experiment carried on with anti-EGFR antibody. Asterisks mark the reported binding sites for Rhi. Fold enrichment for each analysed region is reported on the Y axis as a percentage of the input chromatin (%Input).
Figure 7
Mutations in Cuff affect the subcellular localization of piRNA pathway components. In wild type, Tej, Ago3, Vas and Aub accumulate in the perinuclear nuage (A, C, E and G, respectively). In the cuff mutant, Tej, Ago3, Vas and Aub are dispersed in the cytoplasm, indicating a disruption of the nuage (B, D, F and H). Piwi is normally enriched in the nuclei of nurse cells in wild-type egg chambers (I) and it is not severely affected by mutations in cuff (J). Piwi is prominently nuclear in somatic and germ cells of wild-type germaria (K). In contrast, Piwi is clearly dispersed/downregulated in the germarial germ cells of cuff, vas and aub mutants, while it retains a nuclear accumulation in the somatic cells (L–N, respectively).
Similar articles
- The Drosophila ZAD zinc finger protein Kipferl guides Rhino to piRNA clusters.
Baumgartner L, Handler D, Platzer SW, Yu C, Duchek P, Brennecke J. Baumgartner L, et al. Elife. 2022 Oct 4;11:e80067. doi: 10.7554/eLife.80067. Elife. 2022. PMID: 36193674 Free PMC article. - The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters.
Klattenhoff C, Xi H, Li C, Lee S, Xu J, Khurana JS, Zhang F, Schultz N, Koppetsch BS, Nowosielska A, Seitz H, Zamore PD, Weng Z, Theurkauf WE. Klattenhoff C, et al. Cell. 2009 Sep 18;138(6):1137-49. doi: 10.1016/j.cell.2009.07.014. Epub 2009 Sep 3. Cell. 2009. PMID: 19732946 Free PMC article. - Natural variation of piRNA expression affects immunity to transposable elements.
Ryazansky S, Radion E, Mironova A, Akulenko N, Abramov Y, Morgunova V, Kordyukova MY, Olovnikov I, Kalmykova A. Ryazansky S, et al. PLoS Genet. 2017 Apr 27;13(4):e1006731. doi: 10.1371/journal.pgen.1006731. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28448516 Free PMC article. - The piRNA pathway in flies: highlights and future directions.
Guzzardo PM, Muerdter F, Hannon GJ. Guzzardo PM, et al. Curr Opin Genet Dev. 2013 Feb;23(1):44-52. doi: 10.1016/j.gde.2012.12.003. Epub 2013 Jan 11. Curr Opin Genet Dev. 2013. PMID: 23317515 Free PMC article. Review. - The piRNA pathway in Drosophila ovarian germ and somatic cells.
Sato K, Siomi MC. Sato K, et al. Proc Jpn Acad Ser B Phys Biol Sci. 2020;96(1):32-42. doi: 10.2183/pjab.96.003. Proc Jpn Acad Ser B Phys Biol Sci. 2020. PMID: 31932527 Free PMC article. Review.
Cited by
- Co-dependent Assembly of Drosophila piRNA Precursor Complexes and piRNA Cluster Heterochromatin.
Zhang G, Tu S, Yu T, Zhang XO, Parhad SS, Weng Z, Theurkauf WE. Zhang G, et al. Cell Rep. 2018 Sep 25;24(13):3413-3422.e4. doi: 10.1016/j.celrep.2018.08.081. Cell Rep. 2018. PMID: 30257203 Free PMC article. - Functions of HP1 proteins in transcriptional regulation.
Schoelz JM, Riddle NC. Schoelz JM, et al. Epigenetics Chromatin. 2022 May 7;15(1):14. doi: 10.1186/s13072-022-00453-8. Epigenetics Chromatin. 2022. PMID: 35526078 Free PMC article. Review. - A maternally programmed intergenerational mechanism enables male offspring to make piRNAs from Y-linked precursor RNAs in Drosophila.
Venkei ZG, Gainetdinov I, Bagci A, Starostik MR, Choi CP, Fingerhut JM, Chen P, Balsara C, Whitfield TW, Bell GW, Feng S, Jacobsen SE, Aravin AA, Kim JK, Zamore PD, Yamashita YM. Venkei ZG, et al. Nat Cell Biol. 2023 Oct;25(10):1495-1505. doi: 10.1038/s41556-023-01227-4. Epub 2023 Sep 18. Nat Cell Biol. 2023. PMID: 37723298 Free PMC article. - Diversity of the piRNA pathway for nonself silencing: worm-specific piRNA biogenesis factors.
Izumi N, Tomari Y. Izumi N, et al. Genes Dev. 2014 Apr 1;28(7):665-71. doi: 10.1101/gad.241323.114. Genes Dev. 2014. PMID: 24696451 Free PMC article. - Defining the expression of piRNA and transposable elements in Drosophila ovarian germline stem cells and somatic support cells.
Story B, Ma X, Ishihara K, Li H, Hall K, Peak A, Anoja P, Park J, Haug J, Blanchette M, Xie T. Story B, et al. Life Sci Alliance. 2019 Oct 16;2(5):e201800211. doi: 10.26508/lsa.201800211. Print 2019 Oct. Life Sci Alliance. 2019. PMID: 31619466 Free PMC article.
References
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T (2006) A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442: 203–207 - PubMed
- Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T (2003) The small RNA profile during Drosophila melanogaster development. Dev Cell 5: 337–350 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- P50 GM071508/GM/NIGMS NIH HHS/United States
- R01 GM077620-05/GM/NIGMS NIH HHS/United States
- R01 GM076275/GM/NIGMS NIH HHS/United States
- R01 GM077620-06/GM/NIGMS NIH HHS/United States
- R01 GM077620/GM/NIGMS NIH HHS/United States
- GM076275/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous