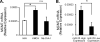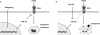miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma - PubMed (original) (raw)
miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma
Nataliya Razumilava et al. Hepatology. 2012 Feb.
Abstract
It has been established that microRNA expression and function contribute to phenotypic features of malignant cells, including resistance to apoptosis. Although targets and functional roles for a number of microRNAs have been described in cholangiocarcinoma, many additional microRNAs dysregulated in this tumor have not been assigned functional roles. In this study, we identify elevated miR-25 expression in malignant cholangiocarcinoma cell lines as well as patient samples. In cultured cells, treatment with the Smoothened inhibitor, cyclopamine, reduced miR-25 expression, suggesting Hedgehog signaling stimulates miR-25 production. Functionally, miR-25 was shown to protect cells against TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Correspondingly, antagonism of miR-25 in culture sensitized cells to apoptotic death. Computational analysis identified the TRAIL Death Receptor-4 (DR4) as a potential novel miR-25 target, and this prediction was confirmed by immunoblot, cell staining, and reporter assays.
Conclusion: These data implicate elevated miR-25 levels in the control of tumor cell apoptosis in cholangiocarcinoma. The identification of the novel miR-25 target DR4 provides a mechanism by which miR-25 contributes to evasion of TRAIL-induced cholangiocarcinoma apoptosis.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures
Figure 1. mir-106b~25 Expression is Supported by Hedgehog Signaling
Quantitation of mir-106b~25 expression in cholangiocarcinoma cells (KMCH) by qRTPCR of total RNA isolated from cell lysates after 24 hr treatment with vehicle, sonic Hedgehog (500 nM), or cyclopamine (5 μM). Expression of studied microRNAs was normalized to RNU-U6 expression (delta-delta Ct). Mean +/- SEM, n = 3, miR-106b and miR-25 expressions were statistically different based on SAM analysis of Hedgehog versus cyclopamine treatment groups, with a false discovery rate of zero.
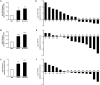
Figure 2. Increased Expression of mir-106b~25 in Cholangiocarcinoma Cell Lines and Human Tumor Samples
Panel A: miR-106b expression was measured by qRT-PCT from total RNA isolated from lysates of the indicated cell lines. Results are presented as relative expression using Z30 as an internal control and employing the delta-delta Ct method (mean +/- SEM; n = 3; *p < 0.05, **p < 0.01). Panel B: miR-93 expression from indicated cell lines, presented as above (***p<0.001). Panel C: miR-25 expression from the indicated cell lines, presented as above. Panel D: Similarly, miR-106b was measured by qRT-PCR of total RNA isolated from frozen human tumor samples. Relative expression was determined (delta-delta Ct) compared to Z30 (internal control) and results are presented as fold change (log2) compared to the average expression measured in four non-malignant human liver samples, and sorted based on expression. Sample numbers indicate the patients from which each was obtained. Panel E: miR-93 expression from human tumor cells, presented as above. Panel F: miR-25 expression from human tumors, presented as above.
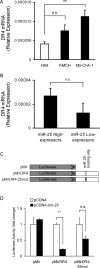
Figure 3. MCM7 mRNA Expression is Increased in Cholangiocarcinoma Cell Lines and Human Samples with High Expression of miR-25
Panel A: Quantitation by qRT-PCR of MCM7 mRNA in whole cell lysates from non-malignant H69 cells compared to malignant KMCH and Mz-ChA-1 cells. Relative expression was determined (delta-delta Ct) compared to 18S (internal control), mean +/-SEM; n = 3; *p < 0.05. Panel B: MCM7 mRNA levels were similarly quantified and analyzed in human cholangiocarcinoma samples comparing groups with high versus low miR-25 expression.
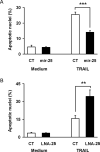
Figure 4. miR-25 Protects Cells from TRAIL-induced Apoptosis
Panel A: Non-malignant H69 cells were transfected with control RNA or mir-25 precursor as indicated to induce miR-25 overexpression. Eighteen hours after transfection, TRAIL was added at 4 ng/ml for an additional 6 hours to induce apoptosis. Cell were stained with DAPI and evaluated based on nuclear morphology for presence of apoptosis. Data are presented as the percentage of cells with apoptotic nuclear morphology out of total cell count and as mean of at least three experiments (mean +/-SEM, ***p<0.001). At least 100 cells were counted in each experiment. Panel B: In parallel, KMCH cells were transfected with control LNA (CT) or miR-25 antagonistic LNA (LNA-25). Two days after transfection, TRAIL at 4 ng/ml was added where indicated for an additional 6 hours. Cells were analyzed with DAPI for percent of apoptotic nuclei, as above (mean +/- SEM, **p<0.01).
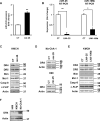
Figure 5. Altered miR-25 Reciprocally Affects DR4 Protein Expression
Panel A: KMCH cells were transfected with mir-25 precursor for 24 hours. Extracted total RNA was analyzed by qRT-PCR to confirm augmented miR-25 expression. Mean +/- SEM; n = 3, **p < 0.01. Panel B: KMCH cells were transfected (48 hr) with locked nucleic acid antagonists to miR-25 (LNA-25), miR-106b (LNA-106b), or control LNA (CT). Total RNA was then subjected to qRT-PCR for miR-25 (left) or miR-106b (right) as well as Z30 (internal control). Data are presented as relative expression (delta-delta Ct compared to Z30). Mean +/- SEM; n = 3, *p<0.05, **p<0.01. Panel C: KMCH total lysates were examined by immunoblot for the indicated polypeptides after cell transfection (24 hr) with control (CT) or mir-25 precursor. Actin was used as a loading control. Apparent molecular weight indicated in kilodaltons (kDa). Panel D: Similarly, Mz-ChA-1 or H69 cells were transfected with control or mir-25 precursor and total cellular protein was analyzed by immunoblot for DR4 and actin. Panel E: KMCH cells, where indicated, were transfected (48 hr) with control LNA (CT), LNA-25, or LNA 106b as a specificity control. Whole cell lysates were analyzed for the indicated polypeptides by immunoblot. Panel F: Total cellular protein was isolated from H69, KMCH, and Mz-ChA-1 cells and analyzed for DR4 protein by immunoblot with actin as a loading control.
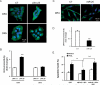
Figure 6. DR4 Repression by miR-25 and Functional Rescue Via miR-25-resistant DR4
Panel A: After transfection of KMCH cells with control locked nucleic acid (CT) or LNA against miR-25 (LNA-25) for 48 hours, slides were prepared and analyzed by confocal microscopy for immunofluorescence intensity of DR4 or DR5. Panel B: Fluorescence intensity of cells transfected and stained as in panel A was quantified by ImageJ software. The data are presented as fold change in the average DR4 or DR5 intensity, mean +/-SEM, ***p<0.001. Panel C: H69 cells were transfected (24hr) with control (CT) or mir-25 precursor; slides were prepared and examined for DR4 immunofluorescence by confocal microscopy. Panel D: DR4 fluorescence intensity in H69 cells was quantified by ImageJ software and presented as fold change in average signal intensity between control and treatment groups, mean +/- SEM, ***p<0.001. Panel E: H69 cells were transfected with GFP plus pCDNA, GFP plus pCDNA-mir-25, or DR4-GFP plus pCDNA-mir-25. Eighteen hours after transfection, TRAIL was added at 4 ng/ml for additional 6 hours to induce apoptosis. Cells were stained with DAPI and GFP-positive cells were evaluated based on nuclear morphology for presence of apoptosis. Data are presented as the percentage of cells with apoptotic nuclear morphology out of total cell count (mean +/-SEM, **p<0.01, ***p<0.001).
Figure 7. miR-25 Targets the DR4 3'UTR to Mediate Gene Expression
Panel A: Total RNA from H69, KMCH, and Mz-ChA-1 cell lines were analyzed for DR4 mRNA expression by qRT-PCR. Relative expression was determined (delta-delta Ct) compared to 18S (internal control), mean +/- SEM; n = 3; *p < 0.05. Panel B: Human tumor samples were grouped based on the level of miR-25 expression (high versus low) and analyzed for DR4 mRNA expression by qRT-PCR. Data are presented as relative expression with 18S used as an internal control. Panel C: Schematic presentation of the empty parental Luciferase reporter (pMir), the luciferase construct containing the full length DR4 3'UTR (pMirDR4), and a two-base binding site mutant (pMirDR4-25mut). Panel D. KMCH cells were transiently transfected with pMir, pMirDR4, or pMirDR4-25mut, as indicated. Cells were also co-transfected with either empty control vector (pCDNA) or with a miR-25 expression vector (pCDNA-mir-25). Twenty-four hours after transfection, cells were lysed and luciferase activity measured. The activity was normalized to the total protein content, and expressed as fold change relative to the corresponding pCDNA value (set at 1.0), mean +/- SEM. ** p < 0.01; n.s. = not significant.
Figure 8. Regulation of Apoptosis by miR-25 Overexpression
Schematic diagram illustrating the role of miR-25 in cholangiocarcinoma TRAIL death-receptor signaling. Panel A. Increased miR-25 expression, driven in part by Hedgehog signaling, targets DR4 for repression which results in TRAIL resistance in tumor cells, permitting cell survival. Panel B. Antagonism of miR-25 via LNA-25 permits elevated DR4 protein levels, and thus increased tumor cell death.
Similar articles
- GLI3-dependent repression of DR4 mediates hedgehog antagonism of TRAIL-induced apoptosis.
Kurita S, Mott JL, Almada LL, Bronk SF, Werneburg NW, Sun SY, Roberts LR, Fernandez-Zapico ME, Gores GJ. Kurita S, et al. Oncogene. 2010 Aug 26;29(34):4848-58. doi: 10.1038/onc.2010.235. Epub 2010 Jun 21. Oncogene. 2010. PMID: 20562908 Free PMC article. - MicroRNA-144 suppresses cholangiocarcinoma cell proliferation and invasion through targeting platelet activating factor acetylhydrolase isoform 1b.
Yang R, Chen Y, Tang C, Li H, Wang B, Yan Q, Hu J, Zou S. Yang R, et al. BMC Cancer. 2014 Dec 5;14:917. doi: 10.1186/1471-2407-14-917. BMC Cancer. 2014. PMID: 25479763 Free PMC article. - miR-128 Targets the SIRT1/ROS/DR5 Pathway to Sensitize Colorectal Cancer to TRAIL-Induced Apoptosis.
Lian B, Yang D, Liu Y, Shi G, Li J, Yan X, Jin K, Liu X, Zhao J, Shang W, Zhang R. Lian B, et al. Cell Physiol Biochem. 2018;49(6):2151-2162. doi: 10.1159/000493818. Epub 2018 Sep 26. Cell Physiol Biochem. 2018. PMID: 30257253 - Emerging Role of microRNA Dysregulation in Diagnosis and Prognosis of Extrahepatic Cholangiocarcinoma.
Prinz C, Frese R, Grams M, Fehring L. Prinz C, et al. Genes (Basel). 2022 Aug 19;13(8):1479. doi: 10.3390/genes13081479. Genes (Basel). 2022. PMID: 36011390 Free PMC article. Review. - The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma.
Sirica AE. Sirica AE. Nat Rev Gastroenterol Hepatol. 2011 Nov 29;9(1):44-54. doi: 10.1038/nrgastro.2011.222. Nat Rev Gastroenterol Hepatol. 2011. PMID: 22143274 Review.
Cited by
- miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets.
Klinge CM. Klinge CM. Mol Cell Endocrinol. 2015 Dec 15;418 Pt 3(0 3):273-97. doi: 10.1016/j.mce.2015.01.035. Epub 2015 Feb 3. Mol Cell Endocrinol. 2015. PMID: 25659536 Free PMC article. Review. - Upregulated MicroRNA-25 Mediates the Migration of Melanoma Cells by Targeting DKK3 through the WNT/β-Catenin Pathway.
Huo J, Zhang Y, Li R, Wang Y, Wu J, Zhang D. Huo J, et al. Int J Mol Sci. 2016 Oct 27;17(11):1124. doi: 10.3390/ijms17111124. Int J Mol Sci. 2016. PMID: 27801786 Free PMC article. - Hedgehog Signaling Modulates Interleukin-33-Dependent Extrahepatic Bile Duct Cell Proliferation in Mice.
Razumilava N, Shiota J, Mohamad Zaki NH, Ocadiz-Ruiz R, Cieslak CM, Zakharia K, Allen BL, Gores GJ, Samuelson LC, Merchant JL. Razumilava N, et al. Hepatol Commun. 2018 Dec 11;3(2):277-292. doi: 10.1002/hep4.1295. eCollection 2019 Feb. Hepatol Commun. 2018. PMID: 30766964 Free PMC article. - miR-25 modulates triacylglycerol and lipid accumulation in goat mammary epithelial cells by repressing PGC-1beta.
Ma L, Qiu H, Chen Z, Li L, Zeng Y, Luo J, Gou D. Ma L, et al. J Anim Sci Biotechnol. 2018 Jun 18;9:48. doi: 10.1186/s40104-018-0262-0. eCollection 2018. J Anim Sci Biotechnol. 2018. PMID: 29946461 Free PMC article. - Clinical significance of circulating miR-25-3p as a novel diagnostic and prognostic biomarker in osteosarcoma.
Fujiwara T, Uotani K, Yoshida A, Morita T, Nezu Y, Kobayashi E, Yoshida A, Uehara T, Omori T, Sugiu K, Komatsubara T, Takeda K, Kunisada T, Kawamura M, Kawai A, Ochiya T, Ozaki T. Fujiwara T, et al. Oncotarget. 2017 May 16;8(20):33375-33392. doi: 10.18632/oncotarget.16498. Oncotarget. 2017. PMID: 28380419 Free PMC article.
References
- Birks DK, Barton VN, Donson AM, Handler MH, Vibhakar R, Foreman NK. Survey of MicroRNA expression in pediatric brain tumors. Pediatric blood & cancer. 2011;56:211–216. - PubMed
- Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. - PubMed
- Dacic S, Kelly L, Shuai Y, Nikiforova MN. miRNA expression profiling of lung adenocarcinomas: correlation with mutational status. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23:1577–1582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007198/DK/NIDDK NIH HHS/United States
- K01 DK079875/DK/NIDDK NIH HHS/United States
- P30DK084567/DK/NIDDK NIH HHS/United States
- P30 DK084567/DK/NIDDK NIH HHS/United States
- K01 DK079875-05/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical