Decreased insulin sensitivity and increased oxidative damage in wasting adipose tissue depots of wild-type mice - PubMed (original) (raw)
Comparative Study
Decreased insulin sensitivity and increased oxidative damage in wasting adipose tissue depots of wild-type mice
Lucila Sackmann-Sala et al. Age (Dordr). 2012 Oct.
Abstract
Unintentional weight loss (wasting) in the elderly is a major health concern as it leads to increased mortality. Several studies have focused on muscle loss, but little is known about the mechanisms giving rise to loss of fat mass at old ages. To investigate potential mechanisms, white adipose tissue (WAT) characteristics and proteomic profiles were compared between adult (10-12-month-old) and aged (22-24-month-old) wild-type mice. Four individual WAT depots were analyzed to account for possible depot-specific differences. Proteomic profiles of WAT depots, along with body weights and compositions, plasma levels of insulin, leptin and adiponectin, insulin tolerance, adipocyte sizes, and products of oxidative damage in each WAT depot were determined. We found that lean mass remained constant while fat mass and insulin tolerance were decreased in old age, as were adipocyte sizes in the WAT depots. Proteomic results showed increased levels of enolase, pyruvate dehydrogenase E1β, NAD(+)-dependent isocitrate dehydrogenase α, and ATP synthase subunit β, and decreased levels of carbonic anhydrase 3 in WAT of aged mice. These data suggest increased aerobic glucose oxidation in wasting WAT, consistent with decreased insulin signaling. Also, Cu/Zn superoxide dismutase and two chaperones were increased in aged WAT depots, indicating higher stress resistance. In agreement, lipid peroxidation (HNE-His adducts) increased in old age, although protein oxidation (carbonyl groups) showed no increase. In conclusion, features of wasting WAT were similar in the four depots, including decreased adipocyte sizes and alterations in protein expression profiles that indicated decreased insulin sensitivity and increased lipid peroxidation.
Figures
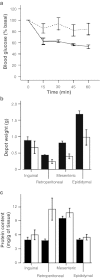
Fig. 1
Insulin tolerance tests (ITTs) and depot-specific weight and protein content of adult and aged mice. Data shown are the mean±SE. a Insulin tolerance was tested on 10-month-old mice (solid line, n = 6) and 22-month-old mice (dotted line, n = 10) and significant effects of age (P = 0.049) were found. b WAT depot weight means for 12-month-old mice (black bars, n = 12) and 24-month-old mice (white bars, n = 14). A two-way ANOVA showed a significant effect of age (P = 0.019), depot (P < 0.001) and age × depot interaction (P = 0.020) for depot weight. c Protein content in WAT depots of 12-month-old mice (black bars, n = 6) and 24-month-old mice (white bars, n = 6). A two-way ANOVA showed a significant effect of depot (P < 0.001) and significant age × depot interaction (P = 0.011) with no effect of age
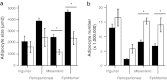
Fig. 2
Age effects in adipocyte size a and number b in the WAT depots. Data shown are the mean±SE and include results obtained from six mice in each age group (except retroperitoneal WAT in the aged group, which could only be analyzed in three mice). Significant differences (P < 0.05) between age groups in each WAT depot (independent _t_-test) are shown with an asterisk. (black bars) 12-month-old mice; (white bars) 24-month-old mice
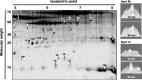
Fig. 3
Representative 2D-gel of WAT showing spots that displayed significant intensity differences (P < 0.01) between age groups (20 spots) or significant interaction of age × depot (four spots, marked with an asterisk). Numbered labels correspond to protein identities shown in Table 2. The right panel shows 3D views of intensity peaks for spots 5b and 12, as examples of age-related changes in WAT. Images were obtained using the 3D viewer tool from PDQuest, which converts spot intensity data to topographical peaks and valleys
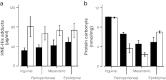
Fig. 4
Oxidative damage products measured in WAT depots of 12- and 24-month-old mice (black and white bars, respectively). Data shown are the mean±SE for five male mice. a HNE-protein adducts were significantly different between age groups (P = 0.021), with no effect of depot or age × depot interaction. b Protein carbonyl content showed no differences between age groups, except for a decrease with age in the retroperitoneal WAT depot (age × depot interaction P < 0.001). On the other hand, differences among depots showed the highest levels in inguinal WAT, followed by retroperitoneal and epididymal WAT, and lowest in the mesenteric depot (P < 0.001)
Similar articles
- Heterogeneity among white adipose tissue depots in male C57BL/6J mice.
Sackmann-Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ. Sackmann-Sala L, et al. Obesity (Silver Spring). 2012 Jan;20(1):101-11. doi: 10.1038/oby.2011.235. Epub 2011 Jul 21. Obesity (Silver Spring). 2012. PMID: 21779095 Free PMC article. - Age-related and depot-specific changes in white adipose tissue of growth hormone receptor-null mice.
Sackmann-Sala L, Berryman DE, Lubbers ER, Zhang H, Vesel CB, Troike KM, Gosney ES, List EO, Kopchick JJ. Sackmann-Sala L, et al. J Gerontol A Biol Sci Med Sci. 2014 Jan;69(1):34-43. doi: 10.1093/gerona/glt110. Epub 2013 Jul 20. J Gerontol A Biol Sci Med Sci. 2014. PMID: 23873966 Free PMC article. - The Gα12/13-coupled receptor LPA4 limits proper adipose tissue expansion and remodeling in diet-induced obesity.
Yanagida K, Igarashi H, Yasuda D, Kobayashi D, Ohto-Nakanishi T, Akahoshi N, Sekiba A, Toyoda T, Ishijima T, Nakai Y, Shojima N, Kubota N, Abe K, Kadowaki T, Ishii S, Shimizu T. Yanagida K, et al. JCI Insight. 2018 Dec 20;3(24):e97293. doi: 10.1172/jci.insight.97293. JCI Insight. 2018. PMID: 30568036 Free PMC article. - Omega-3 fatty acids and adipose tissue biology.
Kuda O, Rossmeisl M, Kopecky J. Kuda O, et al. Mol Aspects Med. 2018 Dec;64:147-160. doi: 10.1016/j.mam.2018.01.004. Epub 2018 Jan 17. Mol Aspects Med. 2018. PMID: 29329795 Review. - Recent advances in the relationship between obesity, inflammation, and insulin resistance.
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Bastard JP, et al. Eur Cytokine Netw. 2006 Mar;17(1):4-12. Eur Cytokine Netw. 2006. PMID: 16613757 Review.
Cited by
- Dynamic differences in oxidative stress and the regulation of metabolism with age in visceral versus subcutaneous adipose.
Liu R, Pulliam DA, Liu Y, Salmon AB. Liu R, et al. Redox Biol. 2015 Dec;6:401-408. doi: 10.1016/j.redox.2015.07.014. Epub 2015 Sep 3. Redox Biol. 2015. PMID: 26355396 Free PMC article. - Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity?
Lubbers ER, List EO, Jara A, Sackman-Sala L, Cordoba-Chacon J, Gahete MD, Kineman RD, Boparai R, Bartke A, Kopchick JJ, Berryman DE. Lubbers ER, et al. J Endocrinol. 2013 Feb 25;216(3):363-74. doi: 10.1530/JOE-12-0505. Print 2013 Mar. J Endocrinol. 2013. PMID: 23261955 Free PMC article. - Oxidative stress and protein carbonylation in adipose tissue - implications for insulin resistance and diabetes mellitus.
Ruskovska T, Bernlohr DA. Ruskovska T, et al. J Proteomics. 2013 Oct 30;92:323-34. doi: 10.1016/j.jprot.2013.04.002. Epub 2013 Apr 11. J Proteomics. 2013. PMID: 23584148 Free PMC article. Review. - Beyond Diabetes: Does Obesity-Induced Oxidative Stress Drive the Aging Process?
Salmon AB. Salmon AB. Antioxidants (Basel). 2016 Jul 18;5(3):24. doi: 10.3390/antiox5030024. Antioxidants (Basel). 2016. PMID: 27438860 Free PMC article. Review. - Oxidative stress in the etiology of age-associated decline in glucose metabolism.
Salmon AB. Salmon AB. Longev Healthspan. 2012 Nov 1;1:7. doi: 10.1186/2046-2395-1-7. eCollection 2012. Longev Healthspan. 2012. PMID: 24764512 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- DK075436-01/DK/NIDDK NIH HHS/United States
- R01 AG019899/AG/NIA NIH HHS/United States
- AG019899-06/AG/NIA NIH HHS/United States
- P01 AG031736/AG/NIA NIH HHS/United States
- R15 DK075436/DK/NIDDK NIH HHS/United States
- 1P01AG031736-01A1/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical