Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease - PubMed (original) (raw)
Review
Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease
Adam R Williams et al. Circ Res. 2011.
Abstract
Mesenchymal stem cells (MSCs) are a prototypical adult stem cell with capacity for self-renewal and differentiation with a broad tissue distribution. Initially described in bone marrow, MSCs have the capacity to differentiate into mesoderm- and nonmesoderm-derived tissues. The endogenous role for MSCs is maintenance of stem cell niches (classically the hematopoietic), and as such, MSCs participate in organ homeostasis, wound healing, and successful aging. From a therapeutic perspective, and facilitated by the ease of preparation and immunologic privilege, MSCs are emerging as an extremely promising therapeutic agent for tissue regeneration. Studies in animal models of myocardial infarction have demonstrated the ability of transplanted MSCs to engraft and differentiate into cardiomyocytes and vasculature cells, recruit endogenous cardiac stem cells, and secrete a wide array of paracrine factors. Together, these properties can be harnessed to both prevent and reverse remodeling in the ischemically injured ventricle. In proof-of-concept and phase I clinical trials, MSC therapy improved left ventricular function, induced reverse remodeling, and decreased scar size. This article reviews the current understanding of MSC biology, mechanism of action in cardiac repair, translational findings, and early clinical trial data of MSC therapy for cardiac disease.
Figures
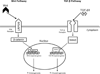
Figure 1. Delivery and potential effects of MSC therapy in cardiac disease
Figure 2. Isolation of mesenchymal stem cells from a bone marrow biopsy
Many stem cells and maturing cells exist in the bone marrow cavity. The mononuclear cells are isolated from red blood cells using Ficoll density centrifugation and the MSCs are separated from the MNCs by plastic adherence in culture.
Figure 3. MSC interactions with immune cells
MSCs are immunoprivileged cells inhibit both innate (neutrophils, dendritic cells, natural killer cells) and adaptive (T cells and B cells) immune cells.
Figure 4. Molecular regulation of MSC differentiation
Wnt signaling and TGF-β induce intracellular signaling regulate differentiation of MSCs.
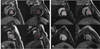
Figure 5. Impact of allogeneic MSC therapy on infarct size
DE-MRI images of swine with chronic ischemic cardiomyopathy (A) injected with 200M allogeneic MSCs before and 3 months after therapy showing a 17% reduction in scar size; and (B) a control animal showing no change in scar size (red arrows indicate gadolinium enhanced scar).
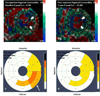
Figure 6. Intramyocardial injection of autologous MSCs improves regional contractility
Tagged CMR strain maps (A) before MSC injection showing a lateral infarct (white arrows) with decreased contractility (red) and (B) post-injection showing improved contractility (green/blue). Corresponding DE-CMR 16 segment model of infarct size (A) before MSC injection and (D) 1 year after injection (less transmularity depicted by decreased orange/yellow). (Panels A and B are reproduced from Williams et al. Circ Res. 2011;108:792–796, courtesy of the American Heart Association)
Similar articles
- Mesenchymal stem cell therapy for heart disease.
Gnecchi M, Danieli P, Cervio E. Gnecchi M, et al. Vascul Pharmacol. 2012 Aug 19;57(1):48-55. doi: 10.1016/j.vph.2012.04.002. Epub 2012 Apr 12. Vascul Pharmacol. 2012. PMID: 22521741 Review. - Application of adipose-derived stem cells in heart disease.
Chen L, Qin F, Ge M, Shu Q, Xu J. Chen L, et al. J Cardiovasc Transl Res. 2014 Oct;7(7):651-63. doi: 10.1007/s12265-014-9585-1. Epub 2014 Sep 10. J Cardiovasc Transl Res. 2014. PMID: 25205213 Review. - Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair.
Oskouei BN, Lamirault G, Joseph C, Treuer AV, Landa S, Da Silva J, Hatzistergos K, Dauer M, Balkan W, McNiece I, Hare JM. Oskouei BN, et al. Stem Cells Transl Med. 2012 Feb;1(2):116-24. doi: 10.5966/sctm.2011-0015. Epub 2012 Feb 7. Stem Cells Transl Med. 2012. PMID: 23197758 Free PMC article. - Mesenchymal stem cells and the treatment of cardiac disease.
Minguell JJ, Erices A. Minguell JJ, et al. Exp Biol Med (Maywood). 2006 Jan;231(1):39-49. doi: 10.1177/153537020623100105. Exp Biol Med (Maywood). 2006. PMID: 16380643 Review. - Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction.
Shafei AE, Ali MA, Ghanem HG, Shehata AI, Abdelgawad AA, Handal HR, Talaat KA, Ashaal AE, El-Shal AS. Shafei AE, et al. J Gene Med. 2017 Dec;19(12). doi: 10.1002/jgm.2995. Epub 2017 Dec 8. J Gene Med. 2017. PMID: 29044850 Review.
Cited by
- Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension.
Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, Kourembanas S. Hansmann G, et al. Pulm Circ. 2012 Apr-Jun;2(2):170-81. doi: 10.4103/2045-8932.97603. Pulm Circ. 2012. PMID: 22837858 Free PMC article. - Ectonucleotidases in solid organ and allogeneic hematopoietic cell transplantation.
Chernogorova P, Zeiser R. Chernogorova P, et al. J Biomed Biotechnol. 2012;2012:208204. doi: 10.1155/2012/208204. Epub 2012 Oct 16. J Biomed Biotechnol. 2012. PMID: 23125523 Free PMC article. Review. - Mesenchymal stem cells improve cardiac conduction by upregulation of connexin 43 through paracrine signaling.
Mureli S, Gans CP, Bare DJ, Geenen DL, Kumar NM, Banach K. Mureli S, et al. Am J Physiol Heart Circ Physiol. 2013 Feb 15;304(4):H600-9. doi: 10.1152/ajpheart.00533.2012. Epub 2012 Dec 15. Am J Physiol Heart Circ Physiol. 2013. PMID: 23241322 Free PMC article. - Matrix Metalloproteinase 1 as a Marker of Tonsil-Derived Mesenchymal Stem Cells to Assess Bone Marrow Cell Migration.
Kim HY, Yoon HS, Lee Y, Kim YH, Cho KA, Woo SY, Kim HS, Ryu KH, Park JW. Kim HY, et al. Tissue Eng Regen Med. 2023 Apr;20(2):271-284. doi: 10.1007/s13770-022-00501-0. Epub 2022 Dec 3. Tissue Eng Regen Med. 2023. PMID: 36462090 Free PMC article. - Meta-analysis of the effect of mesenchymal stem cell transplantation on vascular remodeling after carotid balloon injury in animal models.
Ju X, Zou H, Liu K, Duan J, Li S, Zhou Z, Qi Y, Zhao J, Hu J, Wang L, Jia W, Wei Y, Wang Y, Zhang W, Pang L, Li F. Ju X, et al. PLoS One. 2015 Mar 26;10(3):e0120082. doi: 10.1371/journal.pone.0120082. eCollection 2015. PLoS One. 2015. PMID: 25811171 Free PMC article.
References
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, de SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. - PubMed
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. - PubMed
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. - PubMed
- Lennon DP, Caplan AI. Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1604–1605. - PubMed
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008 - PubMed
Publication types
MeSH terms
Grants and funding
- U54HL081028/HL/NHLBI NIH HHS/United States
- R01 HL107110/HL/NHLBI NIH HHS/United States
- R01 HL110737/HL/NHLBI NIH HHS/United States
- R01 HL094849/HL/NHLBI NIH HHS/United States
- P01HL084275/HL/NHLBI NIH HHS/United States
- U54 HL081028/HL/NHLBI NIH HHS/United States
- P20 HL101443/HL/NHLBI NIH HHS/United States
- HL107110/HL/NHLBI NIH HHS/United States
- HL094849/HL/NHLBI NIH HHS/United States
- HL110737-01/HL/NHLBI NIH HHS/United States
- R01 HL084275/HL/NHLBI NIH HHS/United States
- P20HL101443/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical