Emerging roles of resolvins in the resolution of inflammation and pain - PubMed (original) (raw)
Review
Emerging roles of resolvins in the resolution of inflammation and pain
Ru-Rong Ji et al. Trends Neurosci. 2011 Nov.
Abstract
Resolvins, including D and E series resolvins, are endogenous lipid mediators generated during the resolution phase of acute inflammation from the omega-3 polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Resolvins have potent anti-inflammatory and pro-resolution actions in several animal models of inflammation. Recent findings also demonstrate that resolvin E1 and resolvin D1 can each potently dampen inflammatory and postoperative pain. This review focuses on the mechanisms by which resolvins act on their receptors in immune cells and neurons to normalize exaggerated pain via regulation of inflammatory mediators, transient receptor potential (TRP) ion channels, and spinal cord synaptic transmission. Resolvins may offer novel therapeutic approaches for preventing and treating pain conditions associated with inflammation.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Statement of Conflicts of Interest
C.N.S. may have competing financial interests. Resolvins are biotemplates for stable analogs. Patents on these and their clinical indications are awarded and assigned to the Brigham and Women’s Hospital, and C.N.S. is the inventor. These patents are licensed for clinical development. C.N.S. retains scientific founder stock in Resolvyx Pharmaceutical Company.
Figures
Figure 1
Resolvins and their routes of formation. (a) Omega-3 polyunsaturated fatty acids include DHA and EPA, and are derived from dietary essentially fat (especially enriched in fish). RvD1 and AT-RvD1 are derived from DHA, whereas RvE1 is derived from EPA. Distinct synthetic enzymes, including COX-2, cytochrome P450, and 5- and 15-lipoxygenase are involved in these processes. See Ref [49] for detailed review on the biosynthesis of resolvins. (b) Chemical structures of the resolvins that are discussed in this review.
Figure 2
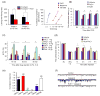
Resolvins (RvE1 and RvD1) inhibit inflammatory and neuropathic pain in mice and suppress TRP channel activity. (a) Mouse model of formalin-induced inflammatory pain. Left panel, summary of the accumulated licking/flicking time of the 1st and 2nd phases. *P < 0.05, vs. vehicle. Right panel, dose-response curves showing the inhibition of formalin-induced 2nd phase pain by intrathecal RvE1, morphine, and NS-398 (a selective COX-2 inhibitor). Note that RvE1, NS-398, and morphine have similar molecular weights. (b) Carrageenan (CRG)-induced heat hyperalgesia is reduced by intraplantar pretreatment of RvE1 and RvD1. *P<0.05, vs. vehicle. (c) CFA-induced heat hyperalgesia on day 3 is differentially reduced by intrathecal administration of RvE1, RvD1, DHA, and EPA. M.P.E, maximum possible effect of anti-hyperalgesia. *P<0.05, vs vehicle. (d) Spinal nerve ligation (SNL)-induced neuropathic pain. SNL-induced heat hyperalgesia on day 1 is reduced by RvE1 and 19-pf-RvE1 (a metabolically stable analogue of RvE1). *P<0.05, vs vehicle. (e) Intracellular Ca2+ responses in HEK293T cells and differential effects of RvD1 (300 nM) on TRP agonist-induced intracellular Ca2+ increases. HEK293T cells were transiently transfected with individual thermoTRP channels and stimulated with appropriate agonists: 0.2 mM capsaicin for TRPV1; 100 mM probenecid for TRPV2; 4 mM camphor for TRPV3; 10 mM 4a-PDD for TRPV4; 300 mM menthol for TRPM8; and 300 mM cinnamaldehyde for TRPA1. **P < 0.01, ***P < 0.001; compared with respective agonist alone. (f) Patch-clamp recordings of traces of spontaneous postsynaptic currents (sEPSCs) in spinal cord slices of mice. Note that the sEPSC frequency after capsaicin perfusion is blocked by RvE1 pretreatment. Reproduced/modified, with permission, from [71] [panels a (right), c–d, and f) and [72] (panels a (left) and e).
Figure 3
The RvE1 receptor ChemR23 is widely expressed in immune cells, glial cells, and neurons in mouse tissues. (a) Double staining of ChmR23 and ED1 shows that ChemR23 is largely colocalized with the macrophage marker ED1 in the dermis of the CFA-inflamed skin. Scale bar: 50 μM. (b) Triple staining in retinal whole-mounts demonstrates that ChemR23 is localized to a subset of colony-stimulating factor-1-receptor–positive (Csf1r+) microglia. Left column, triple staining for lectin (endothelial cells and microglia, red), Csf1r (green) and ChemR23 (magenta). Central panel, merged image (scale bar: 50 μm); white indicates the colocalization of all three stains. Right column, four images of one Csf1r+ cell at indicated focal planes. (c) Double staining of ChemR23 and TRPV1 shows that ChemR23 is largely co-localized with TRPV1 in DRG neurons. Note that ChemR23 is also expressed in satellite glial cells surrounding neurons. Scale bar: 30 μM. (d) ChemR23 staining shows that after ligation of the sciatic nerve ChemR23 is accumulated near the ligation site (arrows), indicating axonal transport of ChemR23. Scale bar: 30 μM. (e) Double staining of ChemR23 and NeuN shows that ChemR23 co-colocalizes with the neuronal marker NeuN in the spinal cord dorsal horn. Scale bar: 50 μM. Note that ChemR23 is also expressed in NeuN-negative glial cells in the spinal cord. Reproduced/modified, with permission, from [61] (panel b), [71] (panels a, c–e).
Figure 4
Schematic of selective actions of resolvins in the resolution of abnormal pain. (a) RvE1 and RvD1 reduce leukotriene B4 functions, NF-κB activation, and infiltration in neutrophils [38]. (b) RvE1 acts on macrophages to induce nonphlogistic phagocytosis of apoptotic neutrophils (Ref [38]). (c) RvE1 and RvD1 inhibit TNF-α and IL-1β expression in microglia (Ref [38, 61]). (d) In peripheral nerve terminals in skin and muscle, RvE1 inhibits TRPV1 activity (Ref [71]), and RvD1 inhibits TRPA1, TRPV3, and TRPV4 activity (Ref [72]). (e) In DRG neuronal cell bodies, RvE1 inhibits capsaicin-induced ERK activation (Ref [71]), and AT-RvD1 inhibits CFA-induced COX-2 and NF-κB and expression (Ref [55]). RvE1 also inhibits TNF-α-induced ERK activation (Ref [71]). (f) At presynaptic sites of spinal cord primary afferent terminals, RvE1 inhibits TRPV1- and TNF-α-induced sEPSC frequency increase. This presumably occurs as a result of reduced glutamate release, via suppression of ERK activation [71]. (g) At postsynaptic sites of spinal cord dorsal horn neurons, RvE1 inhibits TNF-α-induced ERK activation and NMDA receptor hyperactivity (Ref [71]).
Similar articles
- Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1.
Park CK, Xu ZZ, Liu T, Lü N, Serhan CN, Ji RR. Park CK, et al. J Neurosci. 2011 Dec 14;31(50):18433-8. doi: 10.1523/JNEUROSCI.4192-11.2011. J Neurosci. 2011. PMID: 22171045 Free PMC article. - Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis.
Serhan CN, Gotlinger K, Hong S, Arita M. Serhan CN, et al. Prostaglandins Other Lipid Mediat. 2004 Apr;73(3-4):155-72. doi: 10.1016/j.prostaglandins.2004.03.005. Prostaglandins Other Lipid Mediat. 2004. PMID: 15290791 Review. - Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers.
Serhan CN, Arita M, Hong S, Gotlinger K. Serhan CN, et al. Lipids. 2004 Nov;39(11):1125-32. doi: 10.1007/s11745-004-1339-7. Lipids. 2004. PMID: 15726828 Review. - Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1.
Seki H, Tani Y, Arita M. Seki H, et al. Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):126-30. doi: 10.1016/j.prostaglandins.2009.03.002. Epub 2009 Mar 25. Prostaglandins Other Lipid Mediat. 2009. PMID: 19737659 Review.
Cited by
- Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation.
Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, Thomas PG, Dennis EA, Aderem A. Tam VC, et al. Cell. 2013 Jul 3;154(1):213-27. doi: 10.1016/j.cell.2013.05.052. Cell. 2013. PMID: 23827684 Free PMC article. - The FKBP51 Inhibitor SAFit2 Restores the Pain-Relieving C16 Dihydroceramide after Nerve Injury.
Wedel S, Hahnefeld L, Alnouri MW, Offermanns S, Hausch F, Geisslinger G, Sisignano M. Wedel S, et al. Int J Mol Sci. 2022 Nov 17;23(22):14274. doi: 10.3390/ijms232214274. Int J Mol Sci. 2022. PMID: 36430751 Free PMC article. - Resolvin E1 Inhibits Substance P-Induced Potentiation of TRPV1 in Primary Sensory Neurons.
Jo YY, Lee JY, Park CK. Jo YY, et al. Mediators Inflamm. 2016;2016:5259321. doi: 10.1155/2016/5259321. Epub 2016 Sep 25. Mediators Inflamm. 2016. PMID: 27738388 Free PMC article. - Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis.
Chen JJ, Dai L, Zhao LX, Zhu X, Cao S, Gao YJ. Chen JJ, et al. Sci Rep. 2015 May 19;5:10278. doi: 10.1038/srep10278. Sci Rep. 2015. PMID: 25988362 Free PMC article. - Resolvin D2 Relieving Radicular Pain is Associated with Regulation of Inflammatory Mediators, Akt/GSK-3β Signal Pathway and GPR18.
Zhang LY, Liu ZH, Zhu Q, Wen S, Yang CX, Fu ZJ, Sun T. Zhang LY, et al. Neurochem Res. 2018 Dec;43(12):2384-2392. doi: 10.1007/s11064-018-2666-9. Epub 2018 Nov 15. Neurochem Res. 2018. PMID: 30443715
References
- Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. - PubMed
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. - PubMed
- Brennan TJ, et al. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. - PubMed
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054932/NS/NINDS NIH HHS/United States
- R01 CA080153/CA/NCI NIH HHS/United States
- P01 GM095467/GM/NIGMS NIH HHS/United States
- R01 NS067686/NS/NINDS NIH HHS/United States
- R01 NS67686/NS/NINDS NIH HHS/United States
- P01-GM 95467/GM/NIGMS NIH HHS/United States
- R01 DE017794-06/DE/NIDCR NIH HHS/United States
- R01 NS067686-03/NS/NINDS NIH HHS/United States
- R01 DE17794/DE/NIDCR NIH HHS/United States
- R01 DE017794/DE/NIDCR NIH HHS/United States
- NS54932/NS/NINDS NIH HHS/United States
- R01-CA080153/CA/NCI NIH HHS/United States
- R01 NS054932-04/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials