Corridors of migrating neurons in the human brain and their decline during infancy - PubMed (original) (raw)
. 2011 Sep 28;478(7369):382-6.
doi: 10.1038/nature10487.
Thuhien Nguyen, Rebecca A Ihrie, Zaman Mirzadeh, Hui-Hsin Tsai, Michael Wong, Nalin Gupta, Mitchel S Berger, Eric Huang, Jose-Manuel Garcia-Verdugo, David H Rowitch, Arturo Alvarez-Buylla
Affiliations
- PMID: 21964341
- PMCID: PMC3197903
- DOI: 10.1038/nature10487
Corridors of migrating neurons in the human brain and their decline during infancy
Nader Sanai et al. Nature. 2011.
Abstract
The subventricular zone of many adult non-human mammals generates large numbers of new neurons destined for the olfactory bulb. Along the walls of the lateral ventricles, immature neuronal progeny migrate in tangentially oriented chains that coalesce into a rostral migratory stream (RMS) connecting the subventricular zone to the olfactory bulb. The adult human subventricular zone, in contrast, contains a hypocellular gap layer separating the ependymal lining from a periventricular ribbon of astrocytes. Some of these subventricular zone astrocytes can function as neural stem cells in vitro, but their function in vivo remains controversial. An initial report found few subventricular zone proliferating cells and rare migrating immature neurons in the RMS of adult humans. In contrast, a subsequent study indicated robust proliferation and migration in the human subventricular zone and RMS. Here we find that the infant human subventricular zone and RMS contain an extensive corridor of migrating immature neurons before 18 months of age but, contrary to previous reports, this germinal activity subsides in older children and is nearly extinct by adulthood. Surprisingly, during this limited window of neurogenesis, not all new neurons in the human subventricular zone are destined for the olfactory bulb--we describe a major migratory pathway that targets the prefrontal cortex in humans. Together, these findings reveal robust streams of tangentially migrating immature neurons in human early postnatal subventricular zone and cortex. These pathways represent potential targets of neurological injuries affecting neonates.
Figures
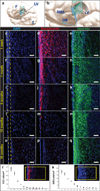
Figure 1. Cytoarchitectural development of the human SVZ during the first 18 months of life
(a, b) Illustrations localizing the horizontal 30µm sections of the anterior ventral SVZ labeled with nuclear marker DAPI (c, f, i, l, o), the immature migrating neuronal marker Doublecortin (DCX) (d, g, j, m, p) and the immature glial marker Vimentin (e, h, k, n, q). F=frontal lobe, T=temporal lobe, LV=lateral ventricle, RMS=rostral migratory stream, OB=olfactory bulb. Large numbers of DCX(+) immature migrating neurons populate the region that develops into the human gap layer (yellow dotted lines). In the first few months of life, radial glia-like Vimentin(+) cells are seen to populate the ventricular lining (e, h), and at 12 months onward, a dense network of Vimentin(+) processes fill the gap area (n, q). DCX and DAPI are co-labeled in the same section (first and second columns), while Vimentin staining is from adjacent sections (third column). (r) DCX(+) immature neurons within the anterior ventral SVZ were quantified in ten 20x fields per section, ten sections per specimen, and 1–3 specimens per timepoint. Each data point represents the mean +/− SD of a single specimen at the designated age (w = week, m = month, y = year). Inset demonstrates the field of quantification (yellow box) for each of the 10 horizontal sections per specimen. (s) Proliferating Ki67(+) subependymal cells were similarly quantified along the anterior ventral human SVZ. Taken together, these data demonstrate, during the first 18 months of life, a sharp decline in proliferating cells and immature migrating neurons coinciding with emergence of the human SVZ gap layer. Scale bars = 30µm.
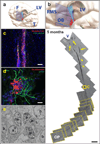
Figure 2. The infant human rostral migratory stream connects the subventricular zone to the olfactory peduncle
Reconstruction of serial sagittal sections of a hematoxylin-stained RMS from inferior lateral ventricle (V) to olfactory peduncle (OP) of an infant specimen (5 months of age). The subventricular source of the proximal RMS shows multiple tributaries of cells (yellow arrows) that appear to coalesce forming the descending (proximal) limb of the RMS. There is no evidence of a continuous ventricular system, but isolated ependymal islets (yellow arrowheads) are observed along the RMS. The distal limb of the RMS moves laterally, out of the sagittal plane of sectioning, as indicated by the yellow section frames. (a, b) Illustrations indicating the anatomic site, plane, and orientation of tissue sectioning, as described above. F=frontal lobe, T=temporal lobe, LV=lateral ventricle, RMS=rostral migratory stream, OB=olfactory bulb. (c) The yellow rectangle indicated along the proximal limb of the RMS demonstrates clusters of DCX(+)PSA-NCAM(+) immature neurons at higher magnification. (d) Individual DCX(+) neuronal chains, seen here in the ventral tip of the anterior horn of a 1-week specimen, are typically embedded in a matrix of GFAP(+) glial processes and often migrate along blood vessels (see Supp. Fig 4d). (e) Ultrastructural analysis of a neuronal chain cross-section identifies immature neurons (type A cells, labeled ‘a’) surrounded by glia (type B cells, labeled ‘b’). Scale bars = 500mm (reconstruction), 50µm (c), and 10µm (d).
Figure 3. Postnatal development and decline of the RMS in the human olfactory tract (OT)
(a) Illustration localizes the anatomical sites for cross-sectional reconstructions of the proximal human OT at (c, i) 1 day, (d, j) 8 months, and (f, k) 7 years. LV=lateral ventricle, RMS=rostral migratory stream, OB=olfactory bulb. High-magnification fields (i, j, k) demonstrate progressive loss of DCX(+)PSA-NCAM(+) immature neurons in the OT. An active RMS is evident at birth and 8 months of age, with many DCX(+)PSA-NCAM(+) cells. By 7 years, however, chains of migrating cells are not observed and DCX(+) cells are rare. (b) Toluidine blue staining of a longitudinal semithin (6µm) section from a 6-month OT demonstrates a central chain of darkened immature neurons surrounded by a matrix of lighter-colored astrocytes. (g) Electron microscopy also demonstrates clusters of type A cells (labeled ‘a’) enmeshed with type B cells within the OT core, including intercellular junctions (yellow arrows) between immature neurons characteristic of chain migration and (h) intermediate filaments (yellow arrowheads) within surrounding Type B cells (labeled ‘b’). Scale bars = 500µm (c, d, f); 75µm (i, j, k); 10µm (b); and 0.5µm (g, h).
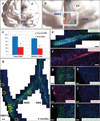
Figure 4. A medial migratory stream (MMS) of immature neurons branches from the proximal rostral migratory stream (RMS) in the infant human brain to supply the ventromedial prefrontal cortex (VMPFC)
(a, b) Illustrations demonstrate the anatomical site of the RMS (blue) and MMS (red). F=Frontal lobe, T=Temporal lobe, LV=lateral ventricle, OB=olfactory bulb. (c) Using cross-sections of the proximal and distal RMS, quantifications of the total number of cells and total number of DCX(+) cells per cross section indicated a significant reduction from proximal to distal RMS. Asterisks indicate p<0.05. (d) Coronal reconstruction of a 6-month specimen reveals a PSA-NCAM(+) medial migratory stream diverging from the RMS to reach the VMPFC. (e) Within this medial migratory stream, chains of PSA-NCAM(+) cells co-express (f) the immature neuronal marker DCX. (g, i, k) Dense clusters of PSA-NCAM(+), Calretinin(+), and Tyrosine Hydroxylase(+) cells are specifically observed within a subregion of the VMPFC, but not at adjacent cortical sites (h, j, l) superior or inferior to this subregion. Scale bars = 150µm (d), 20µm (e–l).
Comment in
- Neural development: Neurogenesis ends near the beginning.
Lewis S. Lewis S. Nat Rev Neurosci. 2011 Oct 20;12(11):616. doi: 10.1038/nrn3128. Nat Rev Neurosci. 2011. PMID: 22011677 No abstract available. - Neuroscience: Gone with the wean.
Arellano JI, Rakic P. Arellano JI, et al. Nature. 2011 Oct 19;478(7369):333-4. doi: 10.1038/478333a. Nature. 2011. PMID: 22012389 No abstract available. - Postnatal neurogenesis in the human forebrain: from two migratory streams to dribbles.
Yang Z, Ming GL, Song H. Yang Z, et al. Cell Stem Cell. 2011 Nov 4;9(5):385-6. doi: 10.1016/j.stem.2011.10.007. Cell Stem Cell. 2011. PMID: 22056132 Free PMC article.
Similar articles
- Expression of ezrin radixin moesin proteins in the adult subventricular zone and the rostral migratory stream.
Persson A, Lindwall C, Curtis MA, Kuhn HG. Persson A, et al. Neuroscience. 2010 May 5;167(2):312-22. doi: 10.1016/j.neuroscience.2010.01.035. Epub 2010 Jan 28. Neuroscience. 2010. PMID: 20109539 - Neurogenesis and neuronal migration in the neonatal rat forebrain anterior subventricular zone do not require GFAP-positive astrocytes.
Law AK, Pencea V, Buck CR, Luskin MB. Law AK, et al. Dev Biol. 1999 Dec 15;216(2):622-34. doi: 10.1006/dbio.1999.9498. Dev Biol. 1999. PMID: 10642797 - RhoE deficiency alters postnatal subventricular zone development and the number of calbindin-expressing neurons in the olfactory bulb of mouse.
Ballester-Lurbe B, González-Granero S, Mocholí E, Poch E, García-Manzanares M, Dierssen M, Pérez-Roger I, García-Verdugo JM, Guasch RM, Terrado J. Ballester-Lurbe B, et al. Brain Struct Funct. 2015 Nov;220(6):3113-30. doi: 10.1007/s00429-014-0846-1. Epub 2014 Jul 10. Brain Struct Funct. 2015. PMID: 25009316 - Architecture and cell types of the adult subventricular zone: in search of the stem cells.
García-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. García-Verdugo JM, et al. J Neurobiol. 1998 Aug;36(2):234-48. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. J Neurobiol. 1998. PMID: 9712307 Review. - Sources and chain migration of neurons to the olfactory bulb.
Woźniak W, Bruska M. Woźniak W, et al. Folia Morphol (Warsz). 1999;58(3 Suppl 2):21-7. Folia Morphol (Warsz). 1999. PMID: 10959258 Review.
Cited by
- αvβ8 integrin adhesion and signaling pathways in development, physiology and disease.
McCarty JH. McCarty JH. J Cell Sci. 2020 Jun 15;133(12):jcs239434. doi: 10.1242/jcs.239434. J Cell Sci. 2020. PMID: 32540905 Free PMC article. Review. - Subventricular zone progenitors in time and space: generating neuronal diversity.
Sequerra EB. Sequerra EB. Front Cell Neurosci. 2014 Dec 22;8:434. doi: 10.3389/fncel.2014.00434. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25565967 Free PMC article. Review. - Emerging insights into the molecular and cellular basis of glioblastoma.
Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A, Brennan C, Ligon KL, Furnari F, Cavenee WK, Depinho RA, Chin L, Hahn WC. Dunn GP, et al. Genes Dev. 2012 Apr 15;26(8):756-84. doi: 10.1101/gad.187922.112. Genes Dev. 2012. PMID: 22508724 Free PMC article. Review. - Tau protein and adult hippocampal neurogenesis.
Fuster-Matanzo A, Llorens-Martín M, Jurado-Arjona J, Avila J, Hernández F. Fuster-Matanzo A, et al. Front Neurosci. 2012 Jul 9;6:104. doi: 10.3389/fnins.2012.00104. eCollection 2012. Front Neurosci. 2012. PMID: 22787440 Free PMC article. - Habilitation Improves Mouse Gait Development Following Neonatal Brain Injury.
Tsuboi Y, Ito A, Otsuka T, Murakami H, Sawada M, Sawamoto K. Tsuboi Y, et al. Prog Rehabil Med. 2022 Dec 1;7:20220061. doi: 10.2490/prm.20220061. eCollection 2022. Prog Rehabil Med. 2022. PMID: 36479304 Free PMC article.
References
- Blakemore WF, Jolly RD. The subependymal plate and associated ependyma in the dog. An ultrastructural study. Journal of neurocytology. 1972;1:69–84. - PubMed
- Perez-Martin M, et al. Ependymal explants from the lateral ventricle of the adult bovine brain: a model system for morphological and functional studies of the ependyma. Cell and tissue research. 2000;300:11–19. - PubMed
- Ponti G, Aimar P, Bonfanti L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. The Journal of comparative neurology. 2006;498:491–507. - PubMed
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Experimental neurology. 2001;172:1–16. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS028478/NS/NINDS NIH HHS/United States
- R01 NS059893/NS/NINDS NIH HHS/United States
- F32 NS058180/NS/NINDS NIH HHS/United States
- R37 HD032116/HD/NICHD NIH HHS/United States
- R01 NS082745/NS/NINDS NIH HHS/United States
- R37 NS028478/NS/NINDS NIH HHS/United States
- R01 HD032116/HD/NICHD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K26 OD010927/OD/NIH HHS/United States
- K26 RR024858/RR/NCRR NIH HHS/United States
- F32 NS 058180/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous