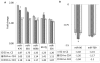Post-transcriptional regulation of IGF1R by key microRNAs in long-lived mutant mice - PubMed (original) (raw)
Post-transcriptional regulation of IGF1R by key microRNAs in long-lived mutant mice
Ruqiang Liang et al. Aging Cell. 2011 Dec.
Abstract
Long-lived mutant mice, both Ames dwarf and growth hormone receptor gene-disrupted or knockout strains, exhibit heightened cognitive robustness and altered IGF1 signaling in the brain. Here, we report, in both these long-lived mice, that three up-regulated lead microRNAs, miR-470, miR-669b, and miR-681, are involved in posttranscriptional regulation of genes pertinent to growth hormone/IGF1 signaling. All three are most prominently localized in the hippocampus and correspond to reduced expression of key IGF1 signaling genes: IGF1, IGF1R, and PI3 kinase. The decline in these genes' expression translates into decreased phosphorylation of downstream molecules AKT and FoxO3a. Cultures transfected with either miR-470, miR-669b, or miR-681 show repressed endogenous expression of all three genes of the IGF1 signaling axis, most significantly IGF1R, while other similarly up-regulated microRNAs, including let-7g and miR-509, do not induce the same levels of repression. Transduction study in IGF1-responsive cell cultures shows significantly reduced IGF1R expression, and AKT to some extent, most notably by miR-681. This is accompanied by decreased levels of downstream phosphorylated forms of AKT and FoxO3a upon IGF1 stimulation. Suppression of IGF1R by the three microRNAs is further validated by IGF1R 3'UTR reporter assays. Taken together, our results suggest that miR-470, miR-669b, and miR-681 are all functionally able to suppress IGF1R and AKT, two upstream genes controlling FoxO3a phosphorylation status. Their up-regulation in growth hormone signaling-deficient mutant mouse brain suggests reduced IGF1 signaling at the posttranscriptional level, for numerous gains of neuronal function in these long-lived mice.
© 2011 University of Louisville. Aging Cell © 2011 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures
Fig. 1. Expression of key miRNAs in Ames dwarf and littermate wild-type mouse brain
A graphical representation of expression levels using qualitative PCR of key miRNAs identified by MM Chips, and respective fold change values within the same age groups among Ames dwarf and littermate controls. D2, D24, D33, C2 and C24: D represents dwarf and C represents littermate WT control mice ages 2, 24 and 33 months respectively. Panel (A) shows key up-regulated miRNAs identified by MM Chips, and panel (B) shows key down-regulated miRNAs. (*p <0.01, **p < 0.0001; all histograms represent average ± std. dev.; n = 3, three samples from each age group of each genotype.)
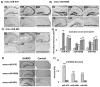
Fig. 2. In situ detection of lead miRNAs in Ames dwarf and GHRKO mouse brains
In situ hybridization (ISH) detection of miRNAs (miR-470, −669b and −681) in brain tissues from Ames dwarf mice (D) at 2, 24, and 33 months, depicted as D2, D24, and D33 respectively, and littermate controls (C) at 2 and 24 months, labeled as C2 and C24 age groups (A, B, C). (D) Increased hybridization signal of these miRNAs in Ames dwarf mice across all age groups, as compared to WT littermates, may be observed. (*p < 0.01, **p < 0.0001; all histograms represent average ± std. dev.; n = 3, three samples from each age group of each genotype.) (E) In situ hybridization (ISH) detection of these three miRNAs in brain tissues from 2 month old GHRKO mice and littermate controls. (F) A graphical representation of densitometric analysis of their expression in cortex and hippocampus is shown. (*p < 0.01, **p < 0.0001; all histograms represent average ± std. dev.; n = 3, three samples from each age group of each genotype.)
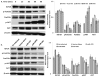
Fig. 3. Altered GH signaling in Ames dwarf and GHRKO mouse brains
In Ames dwarf mouse brain tissues (A), age-dependent decreased levels of IGF1 (14 kDa), IGF1R (95 kDa beta subunit), PI3K(85kDa), pAKT(T308) and pFoxO3a (S253) are observed, compared to littermate WT control mice, but not in total pan-AKT (56 kDa) and FoxO3a (detected as a 100 kDa band). Similar decrease of these proteins in 2 month old GHRKO mouse brain tissues are observed compared to littermate WT controls (B), while total pan-AKT and total FoxO3a are not significantly changed. (C) A graphical representation of densitometric analysis of Western blots of Ames dwarf mouse brain. (*p < 0.01, **p < 0.0001; all histograms represent average ± std. dev.; n = 3, three samples from each age group of each genotype.) The age groups used are Ames dwarf at 2, 24, and 33 months, with D2, D24, and D33 labels, and the wild type control at 2 and 24 months, represented as C2 and C24 respectively. (D) A graphical representation of densitometric analysis of Western blots of GHRKO mouse brain. (*p < 0.01, **p < 0.0001; all histograms represent average ± std. dev.; n = 3, three samples from each age group of each genotype.)
Fig. 4. MiRNA-dependent altered expression of key genes of GH signaling
(A) Western blot analysis of total cell proteins at different time points after serum-deprived WI-38 cells are stimulated by IGF1. As a result of IGF1 stimulation, maximum phosphorylated Akt is seen at 90 minutes. Graphical representation of densitometric data showing the effect of IGF1 signaling at different time points in the form of a histogram is shown with normalized values. β-actin was used as an internal control. (B) Western blot analysis of miRNA (miR-470, −669b and −681) suppression of endogenous expression of GH/IGF axis genes in serum-starved and IGF1-stimulated WI-38 cells. Significant repression of IGF1R (represented by the 95 kDa beta subunit), pAKT (T308) and pFoxO3a (S253) by three key miRNAs (miR-470, −669b, and −681) is observed. No significant change in total AKt expression was observed except in miR-681 transduced cells. No significant change in total FoxO3a expression was observed. Graphical representation of densitometric data in the form of a histogram is shown with normalized values. β-actin was used as an internal control. (*p < 0.01, **p < 0.0001; all histograms represent average ± std. dev.; n = 3, three samples from each of three experiments.)
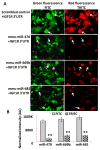
Fig. 5. MiRNA-induced repression of IGF1R 3′UTR in HEK-293 cells
(A) HEK-293 cells co-transfected with one of three key mouse miRNAs (miR-470, −669b, or −681) and IGF1R 3′UTR reporter, showing that the 3′UTR of IGF1R is repressed by the transfected miRNAs, but not by scrambled control and IGF1R 3′UTR co-transfected cells. This indicates that these miRNAs suppress the target (red fluorescence) protein through the 3′UTR of IGF1R. This effect is absent when a plasmid carrying a scrambled sequence is used (indicated with arrows). (B) A graphical representation of densitometric analysis of color intensity is shown. (*p < 0.01, **p < 0.0001; all histograms represent average ± std. dev.; n = 3, three samples from each of three experiments.)
Fig. 6. MiRNA-mediated altered overlapping somatotropic signaling in long-lived mice
This schematic diagram shows the common miRNA-based regulation in both long-lived mouse models (Ames dwarf and GHRKO mice). It depicts the role of three key miRNAs (miR-470, −669b, and −681) targeting a key gene, IGF1R, of the GH/IGF1 axis, and altering GH signaling, thus contributing to longevity.
Similar articles
- IGF1/IGF1R and microRNA let-7e down-regulate each other and modulate proliferation and migration of colorectal cancer cells.
Li Z, Pan W, Shen Y, Chen Z, Zhang L, Zhang Y, Luo Q, Ying X. Li Z, et al. Cell Cycle. 2018;17(10):1212-1219. doi: 10.1080/15384101.2018.1469873. Epub 2018 Jul 18. Cell Cycle. 2018. PMID: 29886785 Free PMC article. - Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles.
Tazearslan C, Huang J, Barzilai N, Suh Y. Tazearslan C, et al. Aging Cell. 2011 Jun;10(3):551-4. doi: 10.1111/j.1474-9726.2011.00697.x. Epub 2011 Apr 12. Aging Cell. 2011. PMID: 21388493 Free PMC article. - Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth.
Disser NP, Sugg KB, Talarek JR, Sarver DC, Rourke BJ, Mendias CL. Disser NP, et al. FASEB J. 2019 Nov;33(11):12680-12695. doi: 10.1096/fj.201901503R. Epub 2019 Sep 16. FASEB J. 2019. PMID: 31536390 Free PMC article. - Upstream and downstream mechanisms for the promoting effects of IGF-1 on differentiation of spermatogonia to primary spermatocytes.
Shen G, Wu R, Liu B, Dong W, Tu Z, Yang J, Xu Z, Pan T. Shen G, et al. Life Sci. 2014 Apr 17;101(1-2):49-55. doi: 10.1016/j.lfs.2014.02.016. Epub 2014 Feb 26. Life Sci. 2014. PMID: 24582811 - Effects and potential mechanisms of IGF1/IGF1R in the liver fibrosis: A review.
Gui R, Li W, Li Z, Wang H, Wu Y, Jiao W, Zhao G, Shen Y, Wang L, Zhang J, Chen S, Hao L, Cheng Y. Gui R, et al. Int J Biol Macromol. 2023 Nov 1;251:126263. doi: 10.1016/j.ijbiomac.2023.126263. Epub 2023 Aug 9. Int J Biol Macromol. 2023. PMID: 37567540 Review.
Cited by
- The role of Foxo3a in neuron-mediated cognitive impairment.
Liu QQ, Wu GH, Wang XC, Xiong XW, Rui-Wang, Yao BL. Liu QQ, et al. Front Mol Neurosci. 2024 Jun 19;17:1424561. doi: 10.3389/fnmol.2024.1424561. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38962803 Free PMC article. Review. - Insulin-Like Growth Factor-1 Signaling in Lung Development and Inflammatory Lung Diseases.
Wang Z, Li W, Guo Q, Wang Y, Ma L, Zhang X. Wang Z, et al. Biomed Res Int. 2018 Jun 19;2018:6057589. doi: 10.1155/2018/6057589. eCollection 2018. Biomed Res Int. 2018. PMID: 30018981 Free PMC article. Review. - The mir-465 family is upregulated with age and attenuates growth hormone signaling in mouse liver.
Elias AE, Kun B, Sabula IMC, Golomb-Mello G, Cespedes Zablah A, Kreiling JA. Elias AE, et al. Aging Cell. 2019 Apr;18(2):e12892. doi: 10.1111/acel.12892. Epub 2019 Jan 13. Aging Cell. 2019. PMID: 30637918 Free PMC article. - Non-coding RNAs as integrators of the effects of age, genes, and environment on ovarian aging.
Cuomo D, Ambrosino C. Cuomo D, et al. Cell Death Dis. 2019 Jan 28;10(2):88. doi: 10.1038/s41419-019-1334-6. Cell Death Dis. 2019. PMID: 30692523 Free PMC article. No abstract available. - mTOR regulates the expression of DNA damage response enzymes in long-lived Snell dwarf, GHRKO, and PAPPA-KO mice.
Dominick G, Bowman J, Li X, Miller RA, Garcia GG. Dominick G, et al. Aging Cell. 2017 Feb;16(1):52-60. doi: 10.1111/acel.12525. Epub 2016 Sep 13. Aging Cell. 2017. PMID: 27618784 Free PMC article.
References
- Aleman A, Torres-Aleman I. Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol. 2009;89:256–265. - PubMed
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG019899/AG/NIA NIH HHS/United States
- R01 AG032290/AG/NIA NIH HHS/United States
- AG19899/AG/NIA NIH HHS/United States
- AG031736/AG/NIA NIH HHS/United States
- AG019899/AG/NIA NIH HHS/United States
- R01 AG019899-10/AG/NIA NIH HHS/United States
- R15 DK075436/DK/NIDDK NIH HHS/United States
- DK075436/DK/NIDDK NIH HHS/United States
- R15 DK075436-01A1/DK/NIDDK NIH HHS/United States
- P01 AG031736-04/AG/NIA NIH HHS/United States
- R01 AG032290-02/AG/NIA NIH HHS/United States
- P01 AG031736/AG/NIA NIH HHS/United States
- P01 AG 031736/AG/NIA NIH HHS/United States
- P01 AG031736-02/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous