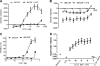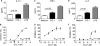CCR8 signaling influences Toll-like receptor 4 responses in human macrophages in inflammatory diseases - PubMed (original) (raw)
CCR8 signaling influences Toll-like receptor 4 responses in human macrophages in inflammatory diseases
Martina Kvist Reimer et al. Clin Vaccine Immunol. 2011 Dec.
Abstract
CCR8 immunity is generally associated with Th2 responses in allergic diseases. In this study, we demonstrate for the first time a pronounced attenuated influx of macrophages in ovalbumin (OVA)-challenged CCR8 knockout mice. To explore whether macrophages in human inflamed lung tissue also were CCR8 positive, human lung tissue from patients with chronic obstructive pulmonary disease (COPD) was evaluated. Indeed, CCR8 expression was pronounced in invading monocytes/macrophages from lungs of patients with Global Initiative for Obstructive Lung Disease (GOLD) stage IV COPD. Given this expression pattern, the functional role of CCR8 on human macrophages was evaluated in vitro. Human peripheral blood monocytes expressed low levels of CCR8, while macrophage colony-stimulating factor (M-CSF)-derived human macrophages expressed significantly elevated surface levels of CCR8. Importantly, CCL1 directly regulated the expression of CD18 and CD49b and hence influenced the adhesion capacity of human macrophages. CCL1 drives chemotaxis in M-CSF-derived macrophages, and this could be completely inhibited by lipopolysaccharide (LPS). Whereas both CCL1 and LPS monotreatment inhibited spontaneous superoxide release in macrophages, CCL1 significantly induced superoxide release in the presence of LPS in a dose-dependent manner. Finally, CCL1 induced production of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) and could inhibit LPS-induced cytokine production in a dose-dependent manner. Our data demonstrate, for the first time, the presence of CCR8 on inflammatory macrophages in human COPD lung tissue. Importantly, the functional data from human macrophages suggest a potential cross talk between the CCR8 and the Toll-like receptor 4 (TLR4) pathways, both of which are present in COPD patients.
Figures
Fig. 1.
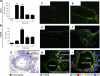
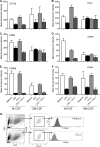
CCR8 and CCL1 expression patterns are regulated in OVA-challenged mice. (A and B) mRNA for CCL1 (A) or CCR8 (B) in mouse lung tissue is upregulated in response to OVA challenge compared to PBS. (C to F) The numbers of CCL1 (C and E)- and CCR8 (D and F)-expressing cells are upregulated in response to OVA challenge (E and F) compared to PBS (C and D). (G and H) EPO staining (eosinophils) of lung tissue at 24 h postchallenge (G) and fluorescent staining with CCR8 (H) show that there is no overlap in expression. (I) Costaining of CCR8 with CD4 shows association of CCR8 with a number of the CD4-positive cells. Each group had 5 animals per experiment, and tissue was evaluated at several locations with similar results. Representative images from one of three experiments with similar results are shown.
Fig. 2.
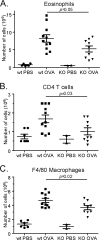
Significant reduction of inflammatory cells in challenged CCR8 knockout mice. The numbers of eosinophils (CCR3 in combination with scatter profile) (A), T cells (CD3 in combination with CD4) (B), and monocytes/macrophages (F4/80 in combination with scatter profile) (C) were all significantly reduced in CCR8 knockout animals compared to wild-type (wt) animals. Results are from one representative out of two experiments showing a similar inhibition of cellular influx. Each symbol represents one animal.
Fig. 3.
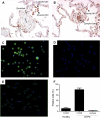
Expression of CCR8 in human COPD lung tissue. (A and B) CCR8 is expressed on endothelial cells, lymphocytes, and alveolar macrophages in human COPD lung tissue. (C and D) Enriched macrophages from human GOLD stage IV COPD lung explant tissue express significant levels of CCR8 (C), whereas the isotype control does not show any staining (D). (E) Enriched macrophages from healthy human lung explants tissue express only low levels of CCR8. (F) The number of CCR8 positive cells was quantified from five patient samples in a flow cytometric analysis.
Fig. 4.
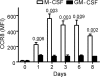
Kinetic analysis of CCR8 expression on M-CSF- or GM-CSF-derived human macrophages. Expression is shown as geometric mean fluorescence intensity (MFI). The standard deviation was routinely less than 20%. Values are means from three independent donors. Data are from one representative out of three experiments.
Fig. 5.
Expression of integrin molecules on human macrophages after treatment with 1 nM CCL1 or 10 ng/ml LPS. (A to F) Expression of CD11b (A), CD18 (B), CD29 (C), CD49b (D), CD49d (E), and CD49f (F) in M-CSF- or GM-CSF-derived macrophages. Expression is shown as geometric mean fluorescence intensity. (G) Results from one representative experiment to show the gating strategy. Viability was monitored in all samples and was routinely >95% (data not shown). The standard deviation was routinely less than 20%. Values are means from three independent donors. Data are from one representative out of three experiments.
Fig. 6.
Macrophage functions are modulated by CCL1 and 10 ng/ml LPS. (A and B) Migration of M-CSF (A)- or GM-CSF (B)-derived macrophages toward increasing concentrations of CCL1 in the presence or absence of LPS. Negative values indicate less migration than for unstimulated cells (i.e., medium control). (C) Inhibition of superoxide generation compared to that with medium alone (i.e., spontaneous superoxide release) in M-CSF- and GM-CSF-derived macrophages. (D) Synergistic agonistic activity on superoxide generation between 10 ng/ml LPS and CCL1 in M-CSF-derived macrophages. The standard deviation was routinely less than 20%. Values are means from three independent donors. Data are from one representative out of three experiments.
Fig. 7.
Production of inflammatory cytokines is modulated by 10 nM CCL1 and 10 ng/ml LPS. Production of IL-1β (A), TNF-α (C), and IL-6 (E) in M-CSF-derived macrophages and inhibition of LPS-induced IL-1β (B), TNF-α (D), and IL-6 (F) by increasing concentrations of CCL1 are shown. Values are means from three independent donors. Data are from one representative out of three experiments.
Similar articles
- Chemokine receptor CCR8 is required for lipopolysaccharide-triggered cytokine production in mouse peritoneal macrophages.
Oshio T, Kawashima R, Kawamura YI, Hagiwara T, Mizutani N, Okada T, Otsubo T, Inagaki-Ohara K, Matsukawa A, Haga T, Kakuta S, Iwakura Y, Hosokawa S, Dohi T. Oshio T, et al. PLoS One. 2014 Apr 8;9(4):e94445. doi: 10.1371/journal.pone.0094445. eCollection 2014. PLoS One. 2014. PMID: 24714157 Free PMC article. - Spinal CCL1/CCR8 signaling interplay as a potential therapeutic target - Evidence from a mouse diabetic neuropathy model.
Zychowska M, Rojewska E, Piotrowska A, Kreiner G, Nalepa I, Mika J. Zychowska M, et al. Int Immunopharmacol. 2017 Nov;52:261-271. doi: 10.1016/j.intimp.2017.09.021. Epub 2017 Oct 12. Int Immunopharmacol. 2017. PMID: 28961489 - Chemokine receptor-8 (CCR8) mediates human vascular smooth muscle cell chemotaxis and metalloproteinase-2 secretion.
Haque NS, Fallon JT, Pan JJ, Taubman MB, Harpel PC. Haque NS, et al. Blood. 2004 Feb 15;103(4):1296-304. doi: 10.1182/blood-2002-05-1480. Epub 2003 Oct 23. Blood. 2004. PMID: 14576057 - CCR8+FOXp3+ Treg cells as master drivers of immune regulation.
Barsheshet Y, Wildbaum G, Levy E, Vitenshtein A, Akinseye C, Griggs J, Lira SA, Karin N. Barsheshet Y, et al. Proc Natl Acad Sci U S A. 2017 Jun 6;114(23):6086-6091. doi: 10.1073/pnas.1621280114. Epub 2017 May 22. Proc Natl Acad Sci U S A. 2017. PMID: 28533380 Free PMC article. - Macrophage heterogeneity in respiratory diseases.
Boorsma CE, Draijer C, Melgert BN. Boorsma CE, et al. Mediators Inflamm. 2013;2013:769214. doi: 10.1155/2013/769214. Epub 2013 Feb 27. Mediators Inflamm. 2013. PMID: 23533311 Free PMC article. Review.
Cited by
- Liposomes Bearing Non-Bilayer Phospholipid Arrangements Induce Specific IgG Anti-Lipid Antibodies by Activating NK1.1+, CD4+ T Cells in Mice.
Landa-Saldívar C, Reséndiz-Mora A, Sánchez-Barbosa S, Sotelo-Rodríguez A, Barrera-Aveleida G, Nevárez-Lechuga I, Galarce-Sosa I, Taniguchi-Ponciano K, Cruz-Guzmán ODR, Wong-Baeza I, Escobar-Gutiérrez A, Baeza I, Wong-Baeza C. Landa-Saldívar C, et al. Membranes (Basel). 2022 Jun 23;12(7):643. doi: 10.3390/membranes12070643. Membranes (Basel). 2022. PMID: 35877846 Free PMC article. - Astroglial TLR9 antagonism promotes chemotaxis and alternative activation of macrophages via modulation of astrocyte-derived signals: implications for spinal cord injury.
Li L, Ni L, Heary RF, Elkabes S. Li L, et al. J Neuroinflammation. 2020 Feb 25;17(1):73. doi: 10.1186/s12974-020-01748-x. J Neuroinflammation. 2020. PMID: 32098620 Free PMC article. - Role of chemokine C-C motif ligand-1 in acute and chronic pulmonary inflammations.
Kishi H, Sato M, Shibata Y, Sato K, Inoue S, Abe S, Kimura T, Nishiwaki M, Yamauchi K, Nemoto T, Igarashi A, Tokairin Y, Nakajima O, Kubota I. Kishi H, et al. Springerplus. 2016 Aug 2;5(1):1241. doi: 10.1186/s40064-016-2904-z. eCollection 2016. Springerplus. 2016. PMID: 27536524 Free PMC article. - Interplay of Chemokines Receptors, Toll-like Receptors, and Host Immunological Pathways.
Chu YT, Liao MT, Tsai KW, Lu KC, Hu WC. Chu YT, et al. Biomedicines. 2023 Aug 25;11(9):2384. doi: 10.3390/biomedicines11092384. Biomedicines. 2023. PMID: 37760825 Free PMC article. Review. - Circulating chemokine ligand levels before and after successful kidney transplantation.
Elmoselhi H, Mansell H, Soliman M, Shoker A. Elmoselhi H, et al. J Inflamm (Lond). 2016 Oct 26;13:32. doi: 10.1186/s12950-016-0141-4. eCollection 2016. J Inflamm (Lond). 2016. PMID: 27795695 Free PMC article.
References
- Bowler R. P., Barnes P. J., Crapo J. D. 2004. The role of oxidative stress in chronic obstructive pulmonary disease. COPD 1:255–277 - PubMed
- Burke W., Fesinmeyer M., Reed K., Hampson L., Carlsten C. 2003. Family history as a predictor of asthma risk. Am. J. Prev. Med. 24:160–169 - PubMed
- Cantor J., Haskins K. 2007. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J. Immunol. 179:5760–5767 - PubMed
- Chiu B. C., Chensue S. W. 2002. Chemokine responses in schistosomal antigen-elicited granuloma formation. Parasite Immunol. 24:285–294 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials