Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms - PubMed (original) (raw)
Comparative Study
Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms
Rodrigo Noseda et al. J Neurosci. 2011.
Abstract
This study identifies massive axonal arbors of trigeminovascular (dura-sensitive) thalamic neurons in multiple cortical areas and proposes a novel framework for conceptualizing migraine headache and its associated symptoms. Individual dura-sensitive neurons identified and characterized electrophysiologically in first-order and higher-order relay thalamic nuclei were juxtacellularly filled with an anterograde tracer that labeled their cell bodies and processes. First-order neurons located in the ventral posteromedial nucleus projected mainly to trigeminal areas of primary (S1) as well as secondary (S2) somatosensory and insular cortices. Higher-order neurons located in the posterior (Po), lateral posterior (LP), and lateral dorsal (LD) nuclei projected to trigeminal and extra-trigeminal areas of S1 and S2, as well as parietal association, retrosplenial, auditory, ectorhinal, motor, and visual cortices. Axonal arbors spread at various densities across most layers of the different cortical areas. Such parallel network of thalamocortical projections may play different roles in the transmission of nociceptive signals from the meninges to the cortex. The findings that individual dura-sensitive Po, LP, and LD neurons project to many functionally distinct and anatomically remote cortical areas extend current thinking on projection patterns of high-order thalamic neurons and position them to relay nociceptive information directly rather than indirectly from one cortical area to another. Such extensive input to diverse cortical areas that are involved in regulation of affect, motor function, visual and auditory perception, spatial orientation, memory retrieval, and olfaction may explain some of the common disturbances in neurological functions during migraine.
Figures
Figure 2.
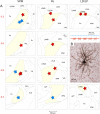
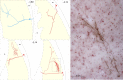
Localization of dura-sensitive (red stars) and dura-insensitive (blue stars) neurons in the thalamus. A, Localization of the cell bodies in the VPM, Po, and LD and LP nuclei (each nucleus highlighted in yellow). Numbers in red indicate the coronal plane (millimeters from bregma) according to the rat brain atlas (Paxinos and Watson, 1998). B, A photomicrograph of the cell body and dendritic tree of a dura-sensitive (LP) neuron that was labeled with the anterograde tracer TMR-dextran. Uptake of the tracer by the target cell body was achieved by inducing bouts of neuronal firing (red trace) in response to repetitive electrical pulses (blue trace) delivered from the recording micropipette, which was preloaded with the tracer. APT, Anterior pretectal nucleus; DLG, dorsal lateral geniculate nucleus; LDDM, laterodorsal thalamic nucleus, dorsomedial; LDVL, laterodorsal thalamic nucleus, ventrolateral; LPMR, lateral posterior thalamic nucleus, mediorostral; Po, posterior thalamic nuclear group; Rt, reticular thalamic nucleus; VPL, ventral posterolateral thalamic nucleus; VPM, ventral posteromedial thalamic nucleus.
Figure 1.
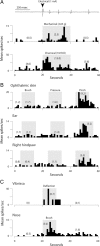
Identification and classification of trigeminovascular thalamic neurons. A, Individual dura-sensitive neurons exhibited discrete discharges in responses to electrical, mechanical, and chemical stimulation of the cranial dura overlying the transverse sinus. B, Dura-sensitive neurons that responded to noxious stimulation of the skin (pinch) were classified as nociceptive if they exhibited no discharge in response to innocuous skin stimuli (brush, pressure). C, Dura-sensitive neurons that responded to gentle mechanical stimulation were classified as non-nociceptive. Stimulus intervals are indicated by gray areas. Numbers in parentheses are spikes per seconds for the corresponding intervals. Bin width, 1 s.
Figure 3.
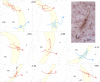
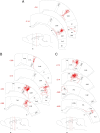
Subcortical mapping of axonal projections and their collaterals. A, Two-dimensional camera lucida reconstruction of cell bodies and dendritic trees of three dura-sensitive thalamocortical neurons, their collateral arbors in the reticular thalamic nucleus (Rt), and caudate–putamen (CPu), and their entry point in the external capsule (ec). B, Localization of the cell bodies (stars) and landmarks of axonal trajectories (circles) of the neurons reconstructed above. Numbers in A and B indicate the coronal plane (millimeters from bregma). Color coding is the same in A and B. For additional abbreviations, see Figure 2.
Figure 4.
Reconstruction of individual axonal arbors in the reticular thalamic nucleus. The parent axons and their trajectories are indicated by arrows. Dura-sensitive neurons are red. Dura-insensitive neurons are blue. The photomicrograph corresponds to the box in bottom left. Numbers indicate the coronal plane (millimeters from bregma). eml, External medullary lamina; ic, internal capsule. For additional abbreviations, see Figure 2.
Figure 5.
Reconstruction of individual axonal arbors in the caudate–putamen. The parent axons and their trajectories are indicated by arrows. Dura-sensitive neurons are red. Dura-insensitive neurons are blue. The photomicrograph corresponds to the box in bottom right. Numbers indicate the coronal plane (millimeters from bregma). ec, External capsule; ic, internal capsule.
Figure 6.
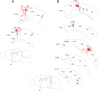
Reconstruction of cortical axonal arbors of individual dura-sensitive VPM (A) and Po (B, C) neurons. Numbers in red indicate the coronal plane (millimeters from bregma). The levels of the different coronal drawings are indicated as red lines in the sagittal drawing of the brain. Note that VPM neurons project mainly to S1, S2, and the insula, whereas Po neurons project to multiple cortical areas outside the so-called pain matrix. Au1, Primary auditory cortex; AuD, secondary auditory cortex, dorsal; AuV, secondary auditory cortex, ventral; Br, bregma; cc, corpus callosum; DI, dysgranular insular cortex; Ect, ectorhinal cortex; GI, granular insular cortex; M1, primary motor cortex; PtA, parietal association cortex; PRh, perirhinal cortex; RSA, retrosplenial cortex; RSGb, retrosplenial granular cortex, b region; S1, primary somatosensory cortex; S1BF, primary somatosensory cortex, barrel field; S1DZ, primary somatosensory cortex, dysgranular; S1FL, primary somatosensory cortex, forelimb; S1HL, primary somatosensory cortex, hindlimb; S1Tr, primary somatosensory cortex, trunk area; S2, secondary somatosensory cortex; V2L, secondary visual cortex, lateral area; V2ML, secondary visual cortex, mediolateral.
Figure 7.
Reconstruction of cortical axonal arbors of individual dura-sensitive LD (A) and LP (B) neurons. Note massive projections to visual and motor cortices. Cg1, Cingulated cortex, area 1; Cg2, cingulated cortex, area 2; M2, secondary motor cortex; V1B, primary visual cortex, binocular; V1M, primary visual cortex, monocular; V2MM, secondary visual cortex, mediomedial; TeA, temporal association cortex. For additional information and abbreviations, see Figure 6.
Figure 8.
Reconstruction of cortical axonal arbors of individual dura-insensitive VPM (A, B) and Po (C) neurons. Note that most terminal arborization was restricted to cortical areas that process somatosensory information. AIP, Agranular insular cortex, posterior; S1ULp, primary somatosensory cortex, upper lip. For additional information, see Figure 6.
Figure 9.
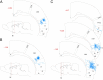
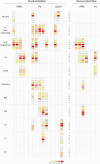
Relative laminar density of terminal arbors of dura-sensitive and dura-insensitive thalamocortical neurons. Fiber density (0–4) is color coded according to the scale at the bottom.
Figure 10.
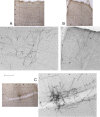
Photomicrographs of axonal arbors in the trigeminal S1 area (S1BF). A, Dura-insensitive VPM neuron. B, C, Dura-sensitive VPM neurons. Roman numerals mark the cortical layers. Immunostained fibers were captured before (monochrome images) and after counterstaining of the tissue with neutral red (color images). Boxed areas in the color images correspond to the higher-power monochrome images. Scale bar, 300 and 100 μm for color and monochrome images, respectively.
Figure 11.
Photomicrographs of axonal arbors in extra-trigeminal S1 areas and S2. A, S1Tr (trunk), dura-sensitive Po neuron. B, S1HL (hindlimb), dura-sensitive Po neuron. C, S2, dura-sensitive VPM neuron. For additional information, including cortical layers and scales, see Figure 10.
Figure 12.
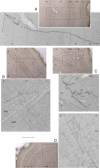
Photomicrographs of axonal arbors in insular, auditory, parietal, ectorhinal, and motor cortices. A, Insula, dura-sensitive VPM neuron. B, PtA and AuD, dura-sensitive Po neuron. C, Ectorhinal, dura-sensitive LP neuron. D, M1/M2, dura-sensitive LD neuron. For additional information, including cortical layers and scales, see Figure 10.
Figure 13.
Photomicrographs of axonal arbors in the visual cortices of a dura-sensitive LP neuron. A, V2MM. B, V1M. C, V2L. For additional information, including cortical layers and scales, see Figure 10.
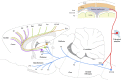
Figure 14.
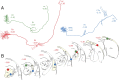
Schematic illustration of the trigeminovascular pathway between the meninges and the cortex. The peripheral limb (meningeal nociceptors) is shown in red. The ascending limb from the spinal trigeminal nucleus (Vc and C1/C2) to the brainstem, thalamus, and hypothalamus is shown in blue. The thalamocortical projections from VPM, PO, and LD/LP are shown in purple, green, and orange, respectively. AH, Anterior hypothalamus; ctg, central tegmental area; LH, lateral hypothalamus; LPO, lateral posterior area; PB, parabrachial area; PH, posterior hypothalamus. For additional abbreviations, see previous figure legends.
Similar articles
- Hypothalamic and basal ganglia projections to the posterior thalamus: possible role in modulation of migraine headache and photophobia.
Kagan R, Kainz V, Burstein R, Noseda R. Kagan R, et al. Neuroscience. 2013 Sep 17;248:359-68. doi: 10.1016/j.neuroscience.2013.06.014. Epub 2013 Jun 25. Neuroscience. 2013. PMID: 23806720 Free PMC article. - Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat.
Berendse HW, Groenewegen HJ. Berendse HW, et al. Neuroscience. 1991;42(1):73-102. doi: 10.1016/0306-4522(91)90151-d. Neuroscience. 1991. PMID: 1713657 - Thalamocortical network interruption: A fresh view for migraine symptoms.
Bolay H. Bolay H. Turk J Med Sci. 2020 Nov 3;50(SI-2):1651-1654. doi: 10.3906/sag-2005-21. Turk J Med Sci. 2020. PMID: 32421284 Free PMC article. Review. - Unitary hypothesis for multiple triggers of the pain and strain of migraine.
Burstein R, Jakubowski M. Burstein R, et al. J Comp Neurol. 2005 Dec 5;493(1):9-14. doi: 10.1002/cne.20688. J Comp Neurol. 2005. PMID: 16258903 Review.
Cited by
- Evidence of Potential Mechanisms of Acupuncture from Functional MRI Data for Migraine Prophylaxis.
Chang CM, Yang CP, Yang CC, Shih PH, Wang SJ. Chang CM, et al. Curr Pain Headache Rep. 2021 May 26;25(7):49. doi: 10.1007/s11916-021-00961-4. Curr Pain Headache Rep. 2021. PMID: 34036477 Review. - Increased Amplitude of Thalamocortical Low-Frequency Oscillations in Patients with Migraine.
Hodkinson DJ, Wilcox SL, Veggeberg R, Noseda R, Burstein R, Borsook D, Becerra L. Hodkinson DJ, et al. J Neurosci. 2016 Jul 27;36(30):8026-36. doi: 10.1523/JNEUROSCI.1038-16.2016. J Neurosci. 2016. PMID: 27466345 Free PMC article. - Dynamic Causal Modelling of the Reduced Habituation to Painful Stimuli in Migraine: An EEG Study.
Bassez I, Van de Steen F, Ricci K, Vecchio E, Gentile E, Marinazzo D, de Tommaso M. Bassez I, et al. Brain Sci. 2020 Oct 7;10(10):712. doi: 10.3390/brainsci10100712. Brain Sci. 2020. PMID: 33036334 Free PMC article. - Investigating the Migraine Cycle over 21 Consecutive Days Using Proton Magnetic Resonance Spectroscopy and Resting-State fMRI: A Pilot Study.
Filippi V, Steiger R, Beliveau V, Frank F, Kaltseis K, Gizewski ER, Broessner G. Filippi V, et al. Brain Sci. 2022 May 14;12(5):646. doi: 10.3390/brainsci12050646. Brain Sci. 2022. PMID: 35625032 Free PMC article. - CGRP and the Trigeminal System in Migraine.
Iyengar S, Johnson KW, Ossipov MH, Aurora SK. Iyengar S, et al. Headache. 2019 May;59(5):659-681. doi: 10.1111/head.13529. Epub 2019 Apr 14. Headache. 2019. PMID: 30982963 Free PMC article. Review.
References
- Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128:932–939. - PubMed
- Angus-Leppan H, Olausson B, Boers P, Lambert GA. Convergence of afferents from superior sagittal sinus and tooth pulp on cells in the thalamus of the cat. Cephalalgia. 1995;15:191–199. - PubMed
- Angus-Leppan H, Lambert GA, Michalicek J. Convergence of occipital nerve and superior sagittal sinus input in the cervical spinal cord of the cat. Cephalalgia. 1997;17:625–630. discussion 623. - PubMed
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–1057. - PubMed
Publication types
MeSH terms
Grants and funding
- NS-069847/NS/NINDS NIH HHS/United States
- R01 NS051484/NS/NINDS NIH HHS/United States
- R01 NS069847/NS/NINDS NIH HHS/United States
- NS-051484/NS/NINDS NIH HHS/United States
- R37 NS079678/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous