Early steps in steroidogenesis: intracellular cholesterol trafficking - PubMed (original) (raw)
Review
. 2011 Dec;52(12):2111-2135.
doi: 10.1194/jlr.R016675. Epub 2011 Oct 5.
Affiliations
- PMID: 21976778
- PMCID: PMC3283258
- DOI: 10.1194/jlr.R016675
Review
Early steps in steroidogenesis: intracellular cholesterol trafficking
Walter L Miller et al. J Lipid Res. 2011 Dec.
Abstract
Steroid hormones are made from cholesterol, primarily derived from lipoproteins that enter cells via receptor-mediated endocytosis. In endo-lysosomes, cholesterol is released from cholesterol esters by lysosomal acid lipase (LAL; disordered in Wolman disease) and exported via Niemann-Pick type C (NPC) proteins (disordered in NPC disease). These diseases are characterized by accumulated cholesterol and cholesterol esters in most cell types. Mechanisms for trans-cytoplasmic cholesterol transport, membrane insertion, and retrieval from membranes are less clear. Cholesterol esters and "free" cholesterol are enzymatically interconverted in lipid droplets. Cholesterol transport to the cholesterol-poor outer mitochondrial membrane (OMM) appears to involve cholesterol transport proteins. Cytochrome P450scc (CYP11A1) then initiates steroidogenesis by converting cholesterol to pregnenolone on the inner mitochondrial membrane (IMM). Acute steroidogenic responses are regulated by cholesterol delivery from OMM to IMM, triggered by the steroidogenic acute regulatory protein (StAR). Chronic steroidogenic capacity is determined by CYP11A1 gene transcription. StAR mutations cause congenital lipoid adrenal hyperplasia, with absent steroidogenesis, potentially lethal salt loss, and 46,XY sex reversal. StAR mutations initially destroy most, but not all steroidogenesis; low levels of StAR-independent steroidogenesis are lost later due to cellular damage, explaining the clinical findings. Rare P450scc mutations cause a similar syndrome. This review addresses these early steps in steroid biosynthesis.
Figures
Fig. 1.
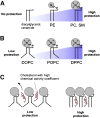
Interactions between cholesterol and membrane lipids, as inferred from model systems in vitro. (A) Lipids that have a large polar head group (N) protect the space between the phospholipids from water. Diacyl glycerol and ceramide lack head groups and, hence, provide no protection; as the size of the head group increases to phosphatidyl ethanolamine (PE) and to phosphatidyl choline (PC) or sphingomyelin (SM), the “umbrella” of protection increases. (B) Bent acyl chains decrease protection. The level of acyl chain saturation alters the shape of the phospholipid; acyl chains containing carbon-carbon double bonds (e.g., dioleoyl phosphatidylcholine, DOPC) create a “bend” in the acyl chain, causing it to take up more space than phospholipids with saturated chains (e.g., palmitoyl-oleoyl-phosphatidylcholine, POPC, or dipalmitoyl phosphatidyl choline, DPPC), affording more exposure to water. (C) Poorly protected cholesterol (e.g., cholesterol in a DOPC-rich bilayer) can readily leave the membrane, so that it has high chemical activity, whereas well-protected cholesterol (e.g., in a membrane rich in phosphatidyl choline) is less readily available. Reproduced from Ref. with permission.
Fig. 2.
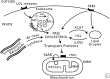
Import and transfer of cellular cholesterol. Human steroidogenic cells take up circulating low-density lipoproteins (LDL) by receptor-mediated endocytosis, directing the cholesterol to endosomes; by contrast, rodent cells bind high-density lipoproteins (HDL) via scavenger receptor B1 (SRB1). Cholesterol can also be synthesized de novo in the endoplasmic reticulum. In endo-lysosomes, cholesterol esters are cleaved by lysosomal acid lipase (LAL) to yield free cholesterol, which is then bound by NPC2, transferred to NPC1, and exported. The MLN64/MENTHO system resides in the same endosomes as the NPC system, but their roles in cholesterol trafficking remain uncertain. Cholesterol can be reesterified by acyl-CoA:cholesterol transferase (ACAT) and stored in lipid droplets as cholesterol esters. Free cholesterol may be produced by hormone-sensitive lipase (HSL), encoded by the LIPE gene. Cholesterol may reach the outer mitochondrial membrane (OMM) by vesicular traffic (not shown) or by nonvesicular means by cholesterol-binding transport proteins, such as those with START domains. Movement of cholesterol from the OMM to the inner mitochondrial membrane (IMM) requires an OMM protein complex (represented by the black box), including the ubiquitously expressed TSPO protein. In the adrenals and gonads, the steroidogenic acute regulatory protein (StAR) is responsible for the rapid movement of cholesterol from the OMM to the IMM, where it can be converted to pregnenolone by the cholesterol side-chain cleavage enzyme P450scc. Copyright © W. L. Miller.
Fig. 3.
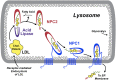
Cell biology of NPC proteins in the endo-lysosome. Circulating LDL is taken up by receptor-mediated endocytosis, and the resulting endosomes fuse with lysosomes containing lysosomal acid lipase (LAL) and NPC proteins. Intra-lysosomal NPC2 binds cholesterol with the side-chain buried in the NPC2 protein and the ester attached to the 3β-hydroxyl group extending into the lysosomal matrix. LAL appears to be able to cleave the ester moieties from the cholesterol either before or after binding to NPC2. NPC2 with bound deesterified cholesterol interacts with the C-terminal cholesterol-binding domain of the NPC1 protein, so that the 3β-hydroxyl group is now buried and the cholesterol side-chain is exposed. The precise mechanism by which NPC1 then inserts the cholesterol into the lysosomal membrane and the means by which that cholesterol is taken up by other proteins remain under investigation. Cholesterol exiting the endo-lysosome may reach the ER or other cellular membranes. Reproduced from Ref. with permission.
Fig. 4.
Structural relationships among MLN64, MENTHO, and StAR. The figure shows the human genes for MLN64 (top), MENTHO (middle), and StAR (bottom) as lines with the numbered boxes representing exons (open boxes, noncoding regions; black boxes, coding regions). The encoded proteins are represented as rectangles with light lines connecting to the corresponding coding exon. Numbers in the protein domains designate amino-acid positions. The four helices of the MENTAL domain of MLN64 and MENTHO that traverse the membrane are represented as gray boxes. The START domains of MLN64 and StAR are indicated by hatched lines. The location of a CpG island in the 5′ region of the MLN64 gene is indicated by a dotted line below exon 1. Reproduced from Ref. with permission.
Fig. 5.
StAR acts on the outer mitochondrial membrane (OMM). The upper panel shows the experimental design (144), depicting the OMM, intramembranous space (IMS), inner mitochondrial membrane (IMM), and various StAR constructs designed to test mitochondrial import and organellar localization. The first 62 residues of StAR, which include the mitochondrial leader and pause sequences, are represented by the corkscrew line, the protease-resistant domain (residues 63-188) by the yellow box, and the protease-sensitive carboxyl-terminal domain by the yellow blob. Mitochondrial protein-import proteins are represented by red ovals. Fusion of N-62 StAR to the C terminus of Tom20, an OMM protein, generates the Tom/StAR construct, which immobilizes StAR on the OMM. Tim9/StAR, a fusion of StAR to an IMS protein, immobilizes StAR in the IMS, and Tim44/StAR, a fusion of StAR to an IMM protein, immobilizes StAR on the matrix side of the IMM. StAR/StAR is a construct that fuses 1-188 StAR to N-62 (63-285) StAR, thus dimerizing the protease-resistant domain (residues 63-188); Del StAR retains residues 1-30 but deletes a “pause” sequence at residues 31-62, and Scc/StAR replaces the StAR leader (either 1-30 or 1-62) with the 39 amino acid leader sequence of human P450scc. The lower panel shows the activity of the constructs illustrated in the upper panel, when transfected into COS-1 cells cotransfected with the F2 fusion of the cholesterol side-chain cleavage system. A low level of StAR-independent steroidogenesis is seen with the vector control, but full-length (1-285) or N-62 StAR increase steroidogenesis 6-fold. The hydroxysterol 22R-OH-cholesterol bypasses the action of StAR, providing an index of the maximal level of steroidogenesis that can be achieved in this system. Transfection with the Tom/StAR fusion or the StAR/StAR dimer achieves this maximal level of steroidogenesis. By contrast, when StAR is placed at the N-terminus rather than the C terminus of Tom20 (StAR/Tom), when it is localized to the matrix side of the IMM (Tim44/StAR), or when it is confined to the IMS (Tim9/StAR), it is inactive. Replacing StAR's leader peptide with that of P450scc also reduces activity. Cell-free transcription/translation linked to mitochondrial import assays show that the level of activity seen with the leader mutants is inversely proportional to their speed of import (144). Copyright © W. L. Miller.
Fig. 6.
Model of N-62 StAR. (Left) Ribbon diagram showing the N terminus in the upper right-hand corner and the C-terminal helix in the lower center, extending out of the plane of the diagram. Residues that contribute to the associations between this C-terminal helix and adjacent structures are shown as ball-and-stick representations: carbon atoms are in white, nitrogen are in blue, oxygen in red, and hydrogen bonds in green. Reprinted from (210) with permission. (Right) Model showing the orientation of StAR as it interacts with the OMM (cover picture of Ref. 210).
Fig. 7.
Two-hit model of lipoid CAH. (A) In a normal human adrenal cell, cholesterol is primarily derived from low-density lipoproteins. The rate-limiting step in steroidogenesis is the flow of cholesterol from the outer to the inner mitochondrial membrane. (B) In early lipoid CAH, StAR-independent mechanisms can still move some cholesterol into the mitochondria, yielding a low level of steroidogenesis. ACTH secretion increases, stimulating further accumulation of cholesterol esters in lipid droplets. (C) As lipid droplets accumulate, they engorge and damage the cell through physical displacement and by the action of cholesterol auto-oxidation products. Steroidogenic capacity is destroyed, and tropic stimulation continues. In the ovary, follicular cells remain unstimulated and undamaged until puberty, when small amounts of estradiol are produced, as in panel B, causing partial feminization, with infertility and hypergonadotropic hypogonadism. Modified from Ref. , with permission.
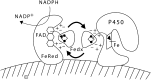
Fig. 8.
Functional organization of mitochondrial P450 enzymes. NADPH first donates electrons to the FAD moiety of ferredoxin reductase (FeRed); positively charged residues in ferredoxin reductase interact with negatively charged residues in ferredoxin (Fedx), permitting the electrons to be transferred to the Fe2S2 center (ball and stick diagram). Ferredoxin then dissociates from ferredoxin reductase and diffuses through the mitochrondrial matrix. The same surface of ferredoxin that received the electrons from ferredoxin reductase then interacts with the redox-partner binding site of a mitochondrial P450, such as P450scc. The electrons from the Fe2S2 center of ferredoxin travel to the heme ring of the P450. The heme iron then mediates catalysis with substrate bound in the P450. Copyright © W. L. Miller.
Similar articles
- Steroid hormone synthesis in mitochondria.
Miller WL. Miller WL. Mol Cell Endocrinol. 2013 Oct 15;379(1-2):62-73. doi: 10.1016/j.mce.2013.04.014. Epub 2013 Apr 28. Mol Cell Endocrinol. 2013. PMID: 23628605 - Disorders in the initial steps of steroid hormone synthesis.
Miller WL. Miller WL. J Steroid Biochem Mol Biol. 2017 Jan;165(Pt A):18-37. doi: 10.1016/j.jsbmb.2016.03.009. Epub 2016 Mar 6. J Steroid Biochem Mol Biol. 2017. PMID: 26960203 Review. - Mitochondrial specificity of the early steps in steroidogenesis.
Miller WL. Miller WL. J Steroid Biochem Mol Biol. 1995 Dec;55(5-6):607-16. doi: 10.1016/0960-0760(95)00212-x. J Steroid Biochem Mol Biol. 1995. PMID: 8547188 Review. - Role of mitochondria in steroidogenesis.
Miller WL. Miller WL. Endocr Dev. 2011;20:1-19. doi: 10.1159/000321204. Epub 2010 Dec 16. Endocr Dev. 2011. PMID: 21164254 Review. - Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR.
Miller WL, Strauss JF 3rd. Miller WL, et al. J Steroid Biochem Mol Biol. 1999 Apr-Jun;69(1-6):131-41. doi: 10.1016/s0960-0760(98)00153-8. J Steroid Biochem Mol Biol. 1999. PMID: 10418987 Review.
Cited by
- Cyclophosphamide-induced testicular injury: the role of chrysin in mitigating iron overload and ferroptosis.
Saleh DO, Abo El Nasr NME, Hussien YA, El-Baset MA, Ahmed KA. Saleh DO, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov 20. doi: 10.1007/s00210-024-03519-4. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39565397 - The effect of lipidomes on the risk of endometrioid endometrial cancer: a Mendelian randomization study.
Lou Y, Jiang F, Guan J. Lou Y, et al. Front Oncol. 2024 Oct 18;14:1436955. doi: 10.3389/fonc.2024.1436955. eCollection 2024. Front Oncol. 2024. PMID: 39493450 Free PMC article. - Primary Adrenal Insufficiency, Complete Sex Reversal, and Unique Clinical Phenotype in a Patient with Severe CYP11A1 (P450scc) Deficiency-Case Report and Literature Overview.
Nowak Z, Preizner-Rzucidło E, Gawlik J, Starzyk JB, Januś D. Nowak Z, et al. Children (Basel). 2024 Oct 12;11(10):1231. doi: 10.3390/children11101231. Children (Basel). 2024. PMID: 39457196 Free PMC article. - Investigation of FF-MAS oxysterole's role in follicular development and its relation to hedgehog signal pathway.
Zırh S, Bahador Zırh E, Erol S, Karakoç Sökmensüer L, Bozdağ G, Müftüoğlu SF. Zırh S, et al. Sci Rep. 2024 Oct 22;14(1):24863. doi: 10.1038/s41598-024-76281-5. Sci Rep. 2024. PMID: 39438722 Free PMC article. - Atypical Imaging Findings in a Cortisol-producing Adrenal Adenoma Predominantly Composed of Lipid-poor Compact Cells.
Kawata S, Obata Y, Akai-Samoto A, Mukai K, Miyashita K, Shimomura I. Kawata S, et al. JCEM Case Rep. 2024 Oct 18;2(11):luae189. doi: 10.1210/jcemcr/luae189. eCollection 2024 Nov. JCEM Case Rep. 2024. PMID: 39430734 Free PMC article.
References
- Jefcoate C. R., McNamara B. C., Artemenko I., Yamazaki T. 1992. Regulation of cholesterol movement to mitochondrial cytochrome P450scc in steroid hormone synthesis. J. Steroid Biochem. Mol. Biol. 43: 751–767. - PubMed
- Spector A. A., Yorek M. A. 1985. Membrane lipid composition and cellular function. J. Lipid Res. 26: 1015–1035. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical