Distinct temporal and anatomical distributions of amyloid-β and tau abnormalities following controlled cortical impact in transgenic mice - PubMed (original) (raw)
Distinct temporal and anatomical distributions of amyloid-β and tau abnormalities following controlled cortical impact in transgenic mice
Hien T Tran et al. PLoS One. 2011.
Abstract
Traumatic brain injury (TBI) is a major environmental risk factor for Alzheimer's disease. Intracellular accumulations of amyloid-β and tau proteins have been observed within hours following severe TBI in humans. Similar abnormalities have been recapitulated in young 3xTg-AD mice subjected to the controlled cortical impact model (CCI) of TBI and sacrificed at 24 h and 7 days post injury. This study investigated the temporal and anatomical distributions of amyloid-β and tau abnormalities from 1 h to 24 h post injury in the same model. Intra-axonal amyloid-β accumulation in the fimbria was detected as early as 1 hour and increased monotonically over 24 hours following injury. Tau immunoreactivity in the fimbria and amygdala had a biphasic time course with peaks at 1 hour and 24 hours, while tau immunoreactivity in the contralateral CA1 rose in a delayed fashion starting at 12 hours after injury. Furthermore, rapid intra-axonal amyloid-β accumulation was similarly observed post controlled cortical injury in APP/PS1 mice, another transgenic Alzheimer's disease mouse model. Acute increases in total and phospho-tau immunoreactivity were also evident in single transgenic Tau(P301L) mice subjected to controlled cortical injury. These data provide further evidence for the causal effects of moderately severe contusional TBI on acceleration of acute Alzheimer-related abnormalities and the independent relationship between amyloid-β and tau in this setting.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
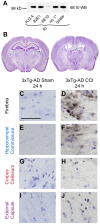
Figure 1. Controlled cortical impact (CCI) causes intra-axonal Aβ accumulation in young 3xTg-AD mice at 24 hours.
A. Immunoprecipitation (IP) and Western blot (WB) showed that HJ3.4 antibody, similar to 82E1 antibody, did not recognize APP, while, 6E10 antibody recognized APP. B. Representative Cresyl violet stained sections showing white matter regions (boxes) with positive axonal Aβ pathology following TBI in 3xTg-AD mice (Modified from [46]). C–J. HJ3.4 staining in uninjured (sham) and injured (CCI) 3xTg-AD mice, counterstained with Cresyl violet. Intra-axonal Aβ accumulation was observed in the ipsilateral fimbria (C–D, black box in B), ipsilateral hippocampal commissure (E–F, blue box in B), ipsilateral corpus callosum (G–H, red box in B) and ipsilateral external capsule (I–J, purple box in B) of injured 3xTg-AD mice. Scale bar in C: 50 µm. Most prominent Aβ staining was observed in the ipsilateral fimbria/fornix of injured mice. Aβ staining has beads-on-a-string and varicose morphologies, consistent with morphologies of injured axons.
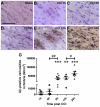
Figure 2. Intra-axonal Aβ accumulation monotonically increases from 1 to 24 hours post CCI in 3xTg-AD mice.
A. Aβ staining with biotinylated HJ3.4 antibody (against Aβ1–13) in the ipsilateral fimbria/fornix of a sham 3xTg-AD mouse. Sections were counterstained with Cresyl violet. Scale bar: 50 µm. B–F. Aβ staining in the ipsilateral fimbria/fornix of an injured 3xTg-AD mouse at 1 h (B), 6 h (C), 9 h (D), 12 h (E) and 24 h (F) after CCI. G. Stereological quantification of total numbers of Aβ-positive axonal varicosities as a function of time after injury in 3xTg-AD mice. N = 4–8 mice per group per time point. Bars represent mean ± SEM. One-way ANOVA with Newman-Keuls post tests, # p<0.05, ## p<0.01: significant increase from injured mice from previous time point. ** p<0.01, *** p<0.0001: significant increase from sham mice at same time point.
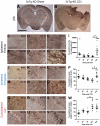
Figure 3. Aβ accumulates in fimbria/fornix axons of 2 month-old APP/PS1 mice at 24 hours post CCI.
A–C. APP staining in the ipsilateral fimbria/fornix of a sham APP/PS1 mouse (A), an injured APP/PS1 mouse (B), and an injured 3xTg-AD mouse (C). Scale bar: 50 µm. Similar extent of axonal injury as detected by APP staining was seen in injured APP/PS1 and 3xTg-AD mice. D–F. Aβ staining with panAβ antibody. G–I. Aβ staining with HJ3.4 antibody. Histological and stereological quantification showed similar extent of Aβ accumulation in injured APP/PS1 and 3xTg-AD mice: 47,257±11,763 HJ3.4-positive varicosities per cubic mm in APP/PS1 (n = 5) vs. 65,437±8,458 in 3xTg-AD mice (n = 8), Student's t-test, p = 0.23.
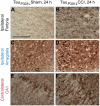
Figure 4. Time course of CCI-induced tau pathology is distinct in several brain structures of 3xTg-AD mice.
Tau staining was with polyclonal total tau antibody. A–B. Tau staining in a sham (A) and an injured (B) 3xTg-AD mouse. Three regions with accelerated tau pathology compared to sham at 24 h post TBI were the ipsilateral fimbria/fornix (black box), ipsilateral amygdala (blue box), and contralateral CA1 (red box). Scale bar: 2 mm. C–H. Higher magnification of punctate tau staining in the ipsilateral fimbria (black box in A–B) of a sham (C) and injured 3xTg-AD mice at 1 h (D), 6 h (E), 9 h (F),12 h (G), and 24 h (H) following CCI. Scale bar in C: 50 µm. I. Stereological quantification of numbers of tau-positive puncta per cubic millimeter in ipsilateral fimbria as a function of time post injury. J–O. Perinuclear, cytoplasmic tau staining in somata of the ipsilateral amygdala (blue box in A–B). P. Stereological quantification of numbers of tau-positive somata per cubic millimeter in the ipsilateral amygdala as a function of time post injury. Q–V. Tau staining in processes of the contralateral CA1 (red box in A–B). W. Stereologcial quantification of tau-positive processes of CA1 pyramidal neurons per cubic millimeter as a function of time post injury. N = 4–8 mice per group per time point. Bars are mean ± SEM in fimbria, and percent of sham ± SEM for amygdala and CA1 region. One-way ANOVA with Newman-Keuls post tests, * p<0.05, ** p<0.01, *** p<0.001: significant increase from sham mice at same time point. # p<0.05: significant increase from injured mice at previous time point.
Figure 5. CCI causes acute axonal tau accumulation and increases somatic tau staining in TauP301L mice.
Polyclonal antibody against total human tau was used for immunohistochemistry. A–B. Punctate tau staining in the ipsilateral fimbria of injured but not sham TauP301L mice. Scale bar in A: 50 µm. C–D. Increased tau immunoreactivity in the cell bodies of the ipsilateral amygdala of injured compared to sham TauP301L mice. E–F. Increased tau immunoreactivity in processes of the contralateral CA1 of injured compared to sham TauP301L mice.
Figure 6. CCI affects tau phosphorylation in the ipsilateral fimbria/fornix of TauP301L mice at 24 hours.
A–B. Phospho-tau staining using pS199 antibody against tau phosphorylated at S199. Scale bar in A: 50 µm. C–D. Phospho-tau staining using PHF1 antibody against tau phosphorylated at S396 and S404. Both phospho-tau antibodies detect punctate axonal tau accumulations in the ipsilateral fimbria of injured but not sham TauP301L mice.
Similar articles
- Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-β accumulation and independently accelerates the development of tau abnormalities.
Tran HT, LaFerla FM, Holtzman DM, Brody DL. Tran HT, et al. J Neurosci. 2011 Jun 29;31(26):9513-25. doi: 10.1523/JNEUROSCI.0858-11.2011. J Neurosci. 2011. PMID: 21715616 Free PMC article. - Inhibition of JNK by a peptide inhibitor reduces traumatic brain injury-induced tauopathy in transgenic mice.
Tran HT, Sanchez L, Brody DL. Tran HT, et al. J Neuropathol Exp Neurol. 2012 Feb;71(2):116-29. doi: 10.1097/NEN.0b013e3182456aed. J Neuropathol Exp Neurol. 2012. PMID: 22249463 Free PMC article. - Human apolipoprotein E4 worsens acute axonal pathology but not amyloid-β immunoreactivity after traumatic brain injury in 3xTG-AD mice.
Bennett RE, Esparza TJ, Lewis HA, Kim E, Mac Donald CL, Sullivan PM, Brody DL. Bennett RE, et al. J Neuropathol Exp Neurol. 2013 May;72(5):396-403. doi: 10.1097/NEN.0b013e31828e24ab. J Neuropathol Exp Neurol. 2013. PMID: 23584199 Free PMC article. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Traumatic brain injury and amyloid-β pathology: a link to Alzheimer's disease?
Johnson VE, Stewart W, Smith DH. Johnson VE, et al. Nat Rev Neurosci. 2010 May;11(5):361-70. doi: 10.1038/nrn2808. Nat Rev Neurosci. 2010. PMID: 20216546 Free PMC article. Review.
Cited by
- Repetitive mild traumatic brain injury affects inflammation and excitotoxic mRNA expression at acute and chronic time-points.
Hiskens MI, Schneiders AG, Vella RK, Fenning AS. Hiskens MI, et al. PLoS One. 2021 May 7;16(5):e0251315. doi: 10.1371/journal.pone.0251315. eCollection 2021. PLoS One. 2021. PMID: 33961674 Free PMC article. - A neurovascular perspective for long-term changes after brain trauma.
Pop V, Badaut J. Pop V, et al. Transl Stroke Res. 2011 Dec 1;2(4):533-45. doi: 10.1007/s12975-011-0126-9. Transl Stroke Res. 2011. PMID: 22350620 Free PMC article. - Brain injury, neuroinflammation and Alzheimer's disease.
Breunig JJ, Guillot-Sestier MV, Town T. Breunig JJ, et al. Front Aging Neurosci. 2013 Jul 11;5:26. doi: 10.3389/fnagi.2013.00026. eCollection 2013. Front Aging Neurosci. 2013. PMID: 23874297 Free PMC article. - Effects of Pyruvate Administration on Mitochondrial Enzymes, Neurological Behaviors, and Neurodegeneration after Traumatic Brain Injury.
Ariyannur PS, Xing G, Barry ES, Benford B, Grunberg NE, Sharma P. Ariyannur PS, et al. Aging Dis. 2021 Jul 1;12(4):983-999. doi: 10.14336/AD.2020.1015. eCollection 2021 Jul. Aging Dis. 2021. PMID: 34221543 Free PMC article. - ReMAPping the microtubule landscape: How phosphorylation dictates the activities of microtubule-associated proteins.
Ramkumar A, Jong BY, Ori-McKenney KM. Ramkumar A, et al. Dev Dyn. 2018 Jan;247(1):138-155. doi: 10.1002/dvdy.24599. Epub 2017 Oct 27. Dev Dyn. 2018. PMID: 28980356 Free PMC article. Review.
References
- Luukinen H, Viramo P, Koski K, Laippala P, Kivela SL. Head injuries and cognitive decline among older adults: a population-based study. Neurology. 1999;52:557–562. - PubMed
- Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, et al. Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. Am J Epidemiol. 1999;149:32–40. - PubMed
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. - PubMed
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, et al. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S28–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous