Rabs and EHDs: alternate modes for traffic control - PubMed (original) (raw)
Review
Rabs and EHDs: alternate modes for traffic control
Jing Zhang et al. Biosci Rep. 2012 Feb.
Abstract
Endocytic trafficking is a highly organized process regulated by a network of proteins, including the Rab family of small GTP-binding proteins and the C-terminal EHDs (Eps15 homology-domain-containing proteins). Central roles for Rab proteins have been described in vesicle budding, delivery, tethering and fusion, whereas little is known about the functions of EHDs in membrane transport. Common effectors for these two protein families have been identified, and they facilitate regulation of sequential steps in transport. By comparing and contrasting key aspects in their modes of function, we shall promote a better understanding of how Rab proteins and EHDs regulate endocytic trafficking.
Figures
Figure 1. Schematic diagram of EHD domain architecture
EHDs contain two helical regions with an ATP-binding G domain in between. The C-terminal EH domain is connected with the helical domain by a 40-residue linker region.
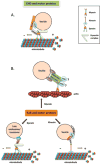
Figure 2. Interaction with motor proteins
(A) EHD and dynein. CRMP2 serves as a link between MICAL-L1-EHD1-associated vesicles and cytoplasmic dynein, and mediates microtubule-dependent vesicle transport. (B) Interactions between Rab proteins and either myosin, kinesin or dynein motors through their respective effectors. Rab11 interacts with myosin Vb through Rab11-FIP2, which is also an EHD-binding partner. Rab5 and its effector hVPS34 (human VPS34) regulate KIF16B (kinesin 16B) transportation of PI(3)P [PtdIns(3)_P_]-positive early endosomes. RILP binds to Rab7 and mediates vesicle transport by recruiting the dynein–dynactin complex.
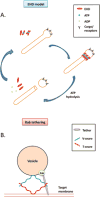
Figure 3. Models of EHDs and Rab proteins
(A) EHD ‘pinchase’ model. EHD-ATP dimers oligomerize along the tubular membrane. ATP hydrolysis induces a conformational change that destabilizes the membrane, which in turn leads to vesicle scission. (B) Rab tethering model. Rabs recruit tethering factors, which in turn interact with SNARE proteins and activate the formation of SNARE complexes, leading to membrane fusion. Scale and stoichiometry are simplified for the illustration.
Similar articles
- Ypt/rab gtpases: regulators of protein trafficking.
Segev N. Segev N. Sci STKE. 2001 Sep 18;2001(100):re11. doi: 10.1126/stke.2001.100.re11. Sci STKE. 2001. PMID: 11579231 Review. - Rab proteins and the compartmentalization of the endosomal system.
Wandinger-Ness A, Zerial M. Wandinger-Ness A, et al. Cold Spring Harb Perspect Biol. 2014 Oct 23;6(11):a022616. doi: 10.1101/cshperspect.a022616. Cold Spring Harb Perspect Biol. 2014. PMID: 25341920 Free PMC article. Review. - Rab GTPases coordinate endocytosis.
Somsel Rodman J, Wandinger-Ness A. Somsel Rodman J, et al. J Cell Sci. 2000 Jan;113 Pt 2:183-92. doi: 10.1242/jcs.113.2.183. J Cell Sci. 2000. PMID: 10633070 Review. - Class I FIPs, Rab11-binding proteins that regulate endocytic sorting and recycling.
Tarbutton E, Peden AA, Junutula JR, Prekeris R. Tarbutton E, et al. Methods Enzymol. 2005;403:512-25. doi: 10.1016/S0076-6879(05)03045-4. Methods Enzymol. 2005. PMID: 16473616 - Rabs and their effectors: achieving specificity in membrane traffic.
Grosshans BL, Ortiz D, Novick P. Grosshans BL, et al. Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):11821-7. doi: 10.1073/pnas.0601617103. Epub 2006 Aug 1. Proc Natl Acad Sci U S A. 2006. PMID: 16882731 Free PMC article. Review.
Cited by
- A missense mutation in Ehd1 associated with defective spermatogenesis and male infertility.
Meindl K, Issler N, Afonso S, Cebrian-Serrano A, Müller K, Sterner C, Othmen H, Tegtmeier I, Witzgall R, Klootwijk E, Davies B, Kleta R, Warth R. Meindl K, et al. Front Cell Dev Biol. 2023 Oct 12;11:1240558. doi: 10.3389/fcell.2023.1240558. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37900275 Free PMC article. - Rab35 and its effectors promote formation of tunneling nanotubes in neuronal cells.
Bhat S, Ljubojevic N, Zhu S, Fukuda M, Echard A, Zurzolo C. Bhat S, et al. Sci Rep. 2020 Oct 8;10(1):16803. doi: 10.1038/s41598-020-74013-z. Sci Rep. 2020. PMID: 33033331 Free PMC article. - Ciliary vesicle formation: a prelude to ciliogenesis.
Yee LE, Reiter JF. Yee LE, et al. Dev Cell. 2015 Mar 23;32(6):665-6. doi: 10.1016/j.devcel.2015.03.012. Dev Cell. 2015. PMID: 25805133 Free PMC article. - Cytomegaloviruses Exploit Recycling Rab Proteins in the Sequential Establishment of the Assembly Compartment.
Lučin P, Kareluša L, Blagojević Zagorac G, Mahmutefendić Lučin H, Pavišić V, Jug Vučko N, Lukanović Jurić S, Marcelić M, Lisnić B, Jonjić S. Lučin P, et al. Front Cell Dev Biol. 2018 Dec 4;6:165. doi: 10.3389/fcell.2018.00165. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30564576 Free PMC article. Review. - Rabankyrin-5 interacts with EHD1 and Vps26 to regulate endocytic trafficking and retromer function.
Zhang J, Reiling C, Reinecke JB, Prislan I, Marky LA, Sorgen PL, Naslavsky N, Caplan S. Zhang J, et al. Traffic. 2012 May;13(5):745-57. doi: 10.1111/j.1600-0854.2012.01334.x. Epub 2012 Feb 20. Traffic. 2012. PMID: 22284051 Free PMC article.
References
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. - PubMed
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. - PubMed
- Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr Opin Cell Biol. 2004;16:392–399. - PubMed
- Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01GM074876/GM/NIGMS NIH HHS/United States
- R01 GM087455/GM/NIGMS NIH HHS/United States
- 1R01GM087455/GM/NIGMS NIH HHS/United States
- P20RR018759/RR/NCRR NIH HHS/United States
- P20 RR018759/RR/NCRR NIH HHS/United States
- R01 GM074876/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous