Acute necrotizing enterocolitis of preterm piglets is characterized by dysbiosis of ileal mucosa-associated bacteria - PubMed (original) (raw)
Acute necrotizing enterocolitis of preterm piglets is characterized by dysbiosis of ileal mucosa-associated bacteria
M Andrea Azcarate-Peril et al. Gut Microbes. 2011 Jul-Aug.
Abstract
Investigation of bacteria involved in pathogenesis of necrotizing enterocolitis (NEC) is limited by infant fragility, analysis restricted to feces, use of culture-based methods, and lack of clinically-relevant animal models. This study used a unique preterm piglet model to characterize spontaneous differences in microbiome composition of NEC-predisposed regions of gut. Preterm piglets (n=23) were cesarean-delivered and nurtured for 30 hours over which time 52% developed NEC. Bacterial DNA from ileal content, ileal mucosa, and colonic mucosa were PCR amplified, subjected to terminal restriction fragment length polymorphism (TRFLP) analysis and targeted 16S rDNA qPCR. Preterm ileal mucosa was specifically bereft in diversity of bacteria compared to ileal content and colonic mucosa. Preterm ileum was restricted to representation by only Proteobacteria, Firmicutes, Cyanobacteria and Chloroflexi. In piglets with NEC, ileal mucosa was uniquely characterized by increases in number of Firmicutes and diversity of phyla to include Actinobacteria and uncultured bacteria. Five specific TRFLP profiles, corresponding in closest identity to Clostridium butyricum, C. neonatale, C. proteolyticum, Streptomyces spp., and Leptolyngbya spp., were significantly more prevalent or observed only among samples from piglets with NEC. Total numbers of Clostridium spp. and C. butyricum were significantly greater in samples of NEC ileal mucosa but not ileal content or colonic mucosa. These results provide strong support for ileal mucosa as a focus for investigation of specific dysbiosis associated with NEC and suggest a significant role for Clostridium spp., and members of the Actinobacteria and Cyanobacteria in the pathogenesis of NEC in preterm piglets.
Figures
Figure 1
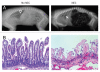
Criteria for diagnosis of NEC in preterm, cesarean-derived and formula-fed piglets. Criteria for NEC included severe abdominal distension, gaseous distention of the intestines (A; closed arrow = gas in portal vein; open arrow = orogastric feeding tube), hemorrhagic discoloration and sloughing or ulceration of the small or large bowel and atrophy, necrosis and sloughing of epithelium (B).
Figure 2
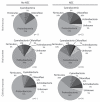
Phylum-associated unique TRFLP profiles expressed as percent of total unique TRFLP profiles identified by disease condition (NEC or No NEC) and sampling site (ileal mucosa, ileal lumen content and colonic mucosa) of piglets. The analysis shows a very highly diverse population of Proteobacteria, represented by the number of unique patterns associated with this phylum, compared to the rest of detected phyla. n = number of piglets.
Figure 3
Principal Coordinate Analysis plots of unique TRFLP patterns as grouped by disease condition (A; 22 no NEC [white] and 27 NEC [black] samples), sex of piglet (B; 36 female [white] and 11 male [black] samples) and sample location (C; 24 ileum [white] and 25 colon [black] mucosa).
Figure 4
Quantitative PCR for total Eubacterial 16s rDNA (A; *p < 0.05 versus ileal mucosa) and Clostridium genus and species-specific 16s rDNA (B; *p < 0.05 versus no NEC). Number of samples = ileal mucosa (no NEC, 11; NEC, 13), ileal content (no NEC, 9; NEC, 10) and colonic mucosa (no NEC, 11; NEC, 14). Data represent median, 10th, 25th, 75th and 90th percentiles.
Similar articles
- Unraveling the Microbiome of Necrotizing Enterocolitis: Insights in Novel Microbial and Metabolomic Biomarkers.
Tarracchini C, Milani C, Longhi G, Fontana F, Mancabelli L, Pintus R, Lugli GA, Alessandri G, Anzalone R, Viappiani A, Turroni F, Mussap M, Dessì A, Cesare Marincola F, Noto A, De Magistris A, Vincent M, Bernasconi S, Picaud JC, Fanos V, Ventura M. Tarracchini C, et al. Microbiol Spectr. 2021 Oct 31;9(2):e0117621. doi: 10.1128/Spectrum.01176-21. Epub 2021 Oct 27. Microbiol Spectr. 2021. PMID: 34704805 Free PMC article. - Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis.
Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, Engstrand L, Lilja HE, Hollister EB, Versalovic J, Neu J. Pammi M, et al. Microbiome. 2017 Mar 9;5(1):31. doi: 10.1186/s40168-017-0248-8. Microbiome. 2017. PMID: 28274256 Free PMC article. Review. - Clostridium butyricum Strains and Dysbiosis Linked to Necrotizing Enterocolitis in Preterm Neonates.
Cassir N, Benamar S, Khalil JB, Croce O, Saint-Faust M, Jacquot A, Million M, Azza S, Armstrong N, Henry M, Jardot P, Robert C, Gire C, Lagier JC, Chabrière E, Ghigo E, Marchandin H, Sartor C, Boutte P, Cambonie G, Simeoni U, Raoult D, La Scola B. Cassir N, et al. Clin Infect Dis. 2015 Oct 1;61(7):1107-15. doi: 10.1093/cid/civ468. Epub 2015 Jun 17. Clin Infect Dis. 2015. PMID: 26084844 - Intraluminal casein model of necrotizing enterocolitis for assessment of mucosal destruction, bacterial translocation, and the effects of allopurinol and N-acetylcysteine.
Koivusalo A, Kauppinen H, Anttila A, Rautelin H, Jusufovic J, Lindahl H, Rintala R. Koivusalo A, et al. Pediatr Surg Int. 2002 Dec;18(8):712-7. doi: 10.1007/s00383-002-0871-7. Epub 2002 Oct 26. Pediatr Surg Int. 2002. PMID: 12598971 Clinical Trial. - Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis.
Hackam DJ, Sodhi CP. Hackam DJ, et al. Cell Mol Gastroenterol Hepatol. 2018 Apr 6;6(2):229-238.e1. doi: 10.1016/j.jcmgh.2018.04.001. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30105286 Free PMC article. Review.
Cited by
- The role of the intestinal microbiota in the pathogenesis of necrotizing enterocolitis.
Grishin A, Papillon S, Bell B, Wang J, Ford HR. Grishin A, et al. Semin Pediatr Surg. 2013 May;22(2):69-75. doi: 10.1053/j.sempedsurg.2013.01.002. Semin Pediatr Surg. 2013. PMID: 23611609 Free PMC article. Review. - Core-, pan- and accessory genome analyses of Clostridium neonatale: insights into genetic diversity.
Mesa V, Monot M, Ferraris L, Popoff M, Mazuet C, Barbut F, Delannoy J, Dupuy B, Butel MJ, Aires J. Mesa V, et al. Microb Genom. 2022 May;8(5):mgen000813. doi: 10.1099/mgen.0.000813. Microb Genom. 2022. PMID: 35550024 Free PMC article. - 16S rRNA gene sequencing, multilocus sequence analysis, and mass spectrometry identification of the proposed new species "Clostridium neonatale".
Bouvet P, Ferraris L, Dauphin B, Popoff MR, Butel MJ, Aires J. Bouvet P, et al. J Clin Microbiol. 2014 Dec;52(12):4129-36. doi: 10.1128/JCM.00477-14. Epub 2014 Sep 17. J Clin Microbiol. 2014. PMID: 25232167 Free PMC article. - Large Animal Models: The Key to Translational Discovery in Digestive Disease Research.
Ziegler A, Gonzalez L, Blikslager A. Ziegler A, et al. Cell Mol Gastroenterol Hepatol. 2016 Nov;2(6):716-724. doi: 10.1016/j.jcmgh.2016.09.003. Cell Mol Gastroenterol Hepatol. 2016. PMID: 28090566 Free PMC article. - Structure and Function of the Fecal Microbiota in Diarrheic Neonatal Piglets.
Yang Q, Huang X, Zhao S, Sun W, Yan Z, Wang P, Li S, Huang W, Zhang S, Liu L, Gun S. Yang Q, et al. Front Microbiol. 2017 Mar 24;8:502. doi: 10.3389/fmicb.2017.00502. eCollection 2017. Front Microbiol. 2017. PMID: 28392784 Free PMC article.
References
- Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:1471–1478. - PubMed
- Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44:1072–1075. - PubMed
- Alfaleh K, Anabrees J, Bassler D. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: a meta-analysis. Neonatology. 2010;97:93–99. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous