Lifespan brain activity, β-amyloid, and Alzheimer's disease - PubMed (original) (raw)
Lifespan brain activity, β-amyloid, and Alzheimer's disease
William J Jagust et al. Trends Cogn Sci. 2011 Nov.
Abstract
Alzheimer's disease (AD) is the most common cause of progressive cognitive decline and dementia in adults. While the amyloid cascade hypothesis of AD posits an initiating role for the β-amyloid (Aβ) protein, there is limited understanding of why Aβ is deposited. A growing body of evidence based on in vitro, animal studies and human imaging work suggests that synaptic activity increases Aβ, which is deposited preferentially in multimodal brain regions that show continuous levels of heightened activation and plasticity across the lifespan. Imaging studies of people with genetic predispositions to AD are consistent with these findings, suggesting a mechanism whereby neural efficiency or cognitive reserve may diminish Aβ deposition. The aggregated findings unify observations from cellular and molecular studies with human cognitive neuroscience to reveal potential mechanisms of AD development.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
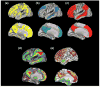
Figure 1. Spatial convergence between Aβ, DMN, heteromodal cortex and cortical hubs
Examination across these panels reveals a high degree of convergence between amyloid distribution, multimodal cortex, cortical hubs and regions utilizing aerobic glycolysis, which is more consistent than the overlap between PIB uptake and the DMN. (a) PIB. Patterns of amyloid deposition in normal elderly controls with slightly elevated PIB values (N=15) are contrasted to low PIB elderly control subjects (N=49; thresholded using clusters at z>1.64 with a cluster significance threshold of p=0.05, corrected; statistical maps were binarized and displayed in Caret v5.6). These slightly elevated subjects have PIB levels below AD but higher than presumably Aβ-free young subjects (N=11; age 20–30), and likely represent the earliest signs of Aβ accumulation (see [44]). (b) DMN. 1 sample t-test of functional connectivity maps of the DMN (using a seed in the posterior cingulate at MNI coordinates 0, −54, 26; N=51; thresholded using clusters at z>3.09 with a cluster significance threshold of p=0.05, corrected; statistical maps were binarized and displayed in Caret v5.6). (c) Heteromodal Cortex. Cortical labeling of heteromodal cortex, adapted from [73]. (d) Hubs. Distribution of cortical hubs. Warmer colors reflect a greater degree of connectivity with other voxels (assessed by counting the number of voxels with a correlation coefficient greater than 0.25 with that specific voxel). Reproduced, with permission, from[16]. (e) Aerobic Glycolysis. Distribution of aerobic glycolysis. Red/yellow colors reflect greater aerobic glycolysis whereas green/blue colors reflect less aerobic glycolysis. Reproduced, with permission, from [18].
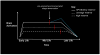
Figure 2. Amyloid and brain activity across the lifespan
Hypothetical trajectories of brain activation across the life span as a function of cognitive reserve (CR) and APOE4. In this model, reserve is associated with increased neuronal efficiency (i.e., lower activation). Consequently, variability in lifelong patterns of brain activation results in different starting points for amyloid accumulation (red line). The blue line represents a ‘secondary’ compensatory response that may be induced by the additional burden imposed by amyloid accumulation, which eventually also fails during a period of decline. This alternative end is possible in all 3 scenarios, but for simplicity is shown only for the low reserve trajectory. These trajectories also describe proposed patterns across different brain regions that vary in their propensity for Aβ deposition. For instance, the high CR trajectory, which displays low levels of activation across the lifespan, is indicative of unimodal regions, whereas the low CR trajectory, which displays high levels of activation across the lifespan, is indicative of multimodal regions.
Similar articles
- Associations between white matter microstructure and amyloid burden in preclinical Alzheimer's disease: A multimodal imaging investigation.
Racine AM, Adluru N, Alexander AL, Christian BT, Okonkwo OC, Oh J, Cleary CA, Birdsill A, Hillmer AT, Murali D, Barnhart TE, Gallagher CL, Carlsson CM, Rowley HA, Dowling NM, Asthana S, Sager MA, Bendlin BB, Johnson SC. Racine AM, et al. Neuroimage Clin. 2014 Feb 19;4:604-14. doi: 10.1016/j.nicl.2014.02.001. eCollection 2014. Neuroimage Clin. 2014. PMID: 24936411 Free PMC article. - Amyloid-β and cognition in aging and Alzheimer's disease: molecular and neurophysiological mechanisms.
Hampel H. Hampel H. J Alzheimers Dis. 2013;33 Suppl 1:S79-86. doi: 10.3233/JAD-2012-129003. J Alzheimers Dis. 2013. PMID: 22531423 Review. - Amyloid-beta and mitochondria in aging and Alzheimer's disease: implications for synaptic damage and cognitive decline.
Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Reddy PH, et al. J Alzheimers Dis. 2010;20 Suppl 2(Suppl 2):S499-512. doi: 10.3233/JAD-2010-100504. J Alzheimers Dis. 2010. PMID: 20413847 Free PMC article. Review. - Alzheimer disease: Aβ-independent processes-rethinking preclinical AD.
Chételat G. Chételat G. Nat Rev Neurol. 2013 Mar;9(3):123-4. doi: 10.1038/nrneurol.2013.21. Epub 2013 Feb 12. Nat Rev Neurol. 2013. PMID: 23399647 Free PMC article. - Inhibition of Drp1 Ameliorates Synaptic Depression, Aβ Deposition, and Cognitive Impairment in an Alzheimer's Disease Model.
Baek SH, Park SJ, Jeong JI, Kim SH, Han J, Kyung JW, Baik SH, Choi Y, Choi BY, Park JS, Bahn G, Shin JH, Jo DS, Lee JY, Jang CG, Arumugam TV, Kim J, Han JW, Koh JY, Cho DH, Jo DG. Baek SH, et al. J Neurosci. 2017 May 17;37(20):5099-5110. doi: 10.1523/JNEUROSCI.2385-16.2017. Epub 2017 Apr 21. J Neurosci. 2017. PMID: 28432138 Free PMC article.
Cited by
- Covarying alterations in Aβ deposition, glucose metabolism, and gray matter volume in cognitively normal elderly.
Oh H, Habeck C, Madison C, Jagust W. Oh H, et al. Hum Brain Mapp. 2014 Jan;35(1):297-308. doi: 10.1002/hbm.22173. Epub 2012 Sep 11. Hum Brain Mapp. 2014. PMID: 22965806 Free PMC article. - Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression.
Huijbers W, Mormino EC, Schultz AP, Wigman S, Ward AM, Larvie M, Amariglio RE, Marshall GA, Rentz DM, Johnson KA, Sperling RA. Huijbers W, et al. Brain. 2015 Apr;138(Pt 4):1023-35. doi: 10.1093/brain/awv007. Epub 2015 Feb 11. Brain. 2015. PMID: 25678559 Free PMC article. - Ginseng gintonin, aging societies, and geriatric brain diseases.
Choi SH, Lee R, Nam SM, Kim DG, Cho IH, Kim HC, Cho Y, Rhim H, Nah SY. Choi SH, et al. Integr Med Res. 2021 Mar;10(1):100450. doi: 10.1016/j.imr.2020.100450. Epub 2020 Jun 13. Integr Med Res. 2021. PMID: 32817818 Free PMC article. Review. - Amyloid Accumulation and Cognitive Decline in Clinically Normal Older Individuals: Implications for Aging and Early Alzheimer's Disease.
Mormino EC, Papp KV. Mormino EC, et al. J Alzheimers Dis. 2018;64(s1):S633-S646. doi: 10.3233/JAD-179928. J Alzheimers Dis. 2018. PMID: 29782318 Free PMC article. Review. - Cognitive reserve and lifestyle: moving towards preclinical Alzheimer's disease.
Arenaza-Urquijo EM, Wirth M, Chételat G. Arenaza-Urquijo EM, et al. Front Aging Neurosci. 2015 Aug 10;7:134. doi: 10.3389/fnagi.2015.00134. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26321944 Free PMC article. Review.
References
- Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. - PubMed
- Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical