Adult murine hippocampal neurogenesis is inhibited by sustained IL-1β and not rescued by voluntary running - PubMed (original) (raw)
Adult murine hippocampal neurogenesis is inhibited by sustained IL-1β and not rescued by voluntary running
Michael D Wu et al. Brain Behav Immun. 2012 Feb.
Abstract
Acute neuroinflammation reduces adult hippocampal neurogenesis but the role of chronic neuroinflammation, which may be more representative of ongoing processes in CNS disorders, remains relatively unknown. Interleukin-1β (IL-1β) is a pro-inflammatory cytokine that has been shown to acutely impair neurogenesis. To further investigate the relationship between sustained IL-1β expression and adult neurogenesis, a mouse model with an IL-1β excisionally activated transgene, IL-1β(XAT), was utilized. Upon exposure to Cre recombinase, IL-1β overexpression in this model results in chronic neuroinflammation, which persists up to 12 months and causes glial activation, cellular recruitment, and deficits in learning and memory. We hypothesized that adult neurogenesis would be reduced by sustained hippocampal IL-1β overexpression and rescued by voluntary running, which has been shown to enhance neurogenesis. Hippocampal inflammation in the IL-1β(XAT) model severely impaired doublecortin (DCX) positive cells at 1 and 3 months after IL-1β induction. Furthermore, BrdU labeling demonstrated a shift in cell lineage from neuronal to astroglial in the context of sustained hippocampal IL-1β overexpression. Deletion of the IL-1 receptor prevented the decrease in DCX(+) cells. Voluntary running did not attenuate the effects of IL-1β expression demonstrated by DCX staining. These results suggest that chronic neuroinflammation severely impairs adult hippocampal neurogenesis and voluntary running is not beneficial as a therapy to rescue these effects.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Figures
Figure 1. Sustained IL-1β overexpression causes focal activation of MHC-II+ cells in the inflamed hippocampus
Staining for MHC-II in the IL-1βXAT model 1 month post-injection of FIV(Cre). Scale bars = 50μm.
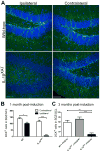
Figure 2. Chronic hippocampal IL-1β overexpression ablates adult neurogenesis
(A) Doublecortin staining (green) and Hoechst-stained nuclei (blue) of hippocampal sections from adult IL-1βXAT and control WT mice one month after unilateral FIV(Cre) injection into the dentate gyrus. Scale bars = 50μm. (B,C) Quantification of DCX+ cells in inflamed hippocampi revealed significant decreases at 1 month and 3 months post-induction. n = 11–15 animals/group, 2-way ANOVA for 1 month, and n = 8–9 animals/group, 1-way ANOVA for 3 months. No significant difference was seen between IL-1βXAT + FIV(GFP) and WT + FIV(Cre) groups (C). Data represent means ± SEM. * = p<0.05, ** = p<0.01, *** = p<0.001.
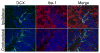
Figure 3. Staining for activated Iba-1+ microglia as well as DCX+ cells in the presence of sustained IL-1β expression
FIV(Cre)-injected hippocampus of IL-1βXAT showed robust microglial activation as shown by increased numbers of Iba-1+ cells with thicker processes 1 month after injection. No direct association with DCX+ cells was seen. Iba-1 = red, DCX = green, Hoechst-stained nuclei = blue. Scale bars = 5μm.
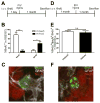
Figure 4. Newborn cells in the SGZ fail to develop into neurons and instead become astroglia in the context of IL-1β overexpression
Experimental design for pulse-labeling with intracerebroventricular BrdU (A,D). IL-1βXAT mice received two intrahippocampal injections, one on each side: FIV(Cre) and FIV(lacZ). Quantification of BrdU+ cells labeled 29 days and 8 weeks previously that colocalize with neuronal (NeuN) and astroglial (GFAP) markers (B,E). Representative immunofluorescent images of BrdU+GFAP+ cells and BrdU+NeuN+ cells (C,F). Images taken at 60X. n = 4 animals per time point, 2-way ANOVA or Student’s t-test. Data represent means ± SEM. * = p<0.05, *** = p<0.001.
Figure 5. The negative effect of IL-1β on DCX+ cells is dependent on the type 1 IL-1 receptor
Quantification of DCX+ cells from FIV(Cre)-injected IL-1βXAT animals 2 weeks post-injection that are wild-type (+/+), heterozygote (+/−), or knockouts (−/−) for the type 1 IL-1 receptor, n = 3 animals/group, 2-way ANOVA. Data represent means ± SEM. ** = p<0.01, *** = p<0.001.
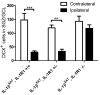
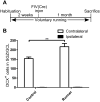
Figure 6. Voluntary running does not alleviate the detrimental effect of IL-1β on DCX+ cells in the SGZ
Experimental design for IL-1βXAT animals that were given access to non-functional (controls) or functional running wheels for 6 weeks prior to sacrifice (A). Quantification of DCX staining demonstrated that voluntary running increased cell numbers only on the contralateral, uninjected hippocampi (B). n = 6–8 animals/group, 2-way ANOVA. Data are means ± SEM. ** = p<0.01.
Similar articles
- Sustained IL-1β expression impairs adult hippocampal neurogenesis independent of IL-1 signaling in nestin+ neural precursor cells.
Wu MD, Montgomery SL, Rivera-Escalera F, Olschowka JA, O'Banion MK. Wu MD, et al. Brain Behav Immun. 2013 Aug;32:9-18. doi: 10.1016/j.bbi.2013.03.003. Epub 2013 Mar 16. Brain Behav Immun. 2013. PMID: 23510988 Free PMC article. - Behavioral, structural and molecular changes following long-term hippocampal IL-1β overexpression in transgenic mice.
Hein AM, Zarcone TJ, Parfitt DB, Matousek SB, Carbonari DM, Olschowka JA, O'Banion MK. Hein AM, et al. J Neuroimmune Pharmacol. 2012 Mar;7(1):145-55. doi: 10.1007/s11481-011-9294-3. Epub 2011 Jul 12. J Neuroimmune Pharmacol. 2012. PMID: 21748283 Free PMC article. - Chronic intestinal inflammation alters hippocampal neurogenesis.
Zonis S, Pechnick RN, Ljubimov VA, Mahgerefteh M, Wawrowsky K, Michelsen KS, Chesnokova V. Zonis S, et al. J Neuroinflammation. 2015 Apr 3;12:65. doi: 10.1186/s12974-015-0281-0. J Neuroinflammation. 2015. PMID: 25889852 Free PMC article. - Behavioral deficits induced by third-trimester equivalent alcohol exposure in male C57BL/6J mice are not associated with reduced adult hippocampal neurogenesis but are still rescued with voluntary exercise.
Hamilton GF, Bucko PJ, Miller DS, DeAngelis RS, Krebs CP, Rhodes JS. Hamilton GF, et al. Behav Brain Res. 2016 Nov 1;314:96-105. doi: 10.1016/j.bbr.2016.07.052. Epub 2016 Aug 1. Behav Brain Res. 2016. PMID: 27491590 Free PMC article. - Nuclear deterrents: Intrinsic regulators of IL-1β-induced effects on hippocampal neurogenesis.
O'Léime CS, Cryan JF, Nolan YM. O'Léime CS, et al. Brain Behav Immun. 2017 Nov;66:394-412. doi: 10.1016/j.bbi.2017.07.153. Epub 2017 Jul 24. Brain Behav Immun. 2017. PMID: 28751020 Review.
Cited by
- Vaccination reduces central nervous system IL-1β and memory deficits after COVID-19 in mice.
Vanderheiden A, Hill JD, Jiang X, Deppen B, Bamunuarachchi G, Soudani N, Joshi A, Cain MD, Boon ACM, Klein RS. Vanderheiden A, et al. Nat Immunol. 2024 Jul;25(7):1158-1171. doi: 10.1038/s41590-024-01868-z. Epub 2024 Jun 20. Nat Immunol. 2024. PMID: 38902519 - Binge ethanol exposure in advanced age elevates neuroinflammation and early indicators of neurodegeneration and cognitive impairment in female mice.
Anton PE, Rutt LN, Kaufman ML, Busquet N, Kovacs EJ, McCullough RL. Anton PE, et al. Brain Behav Immun. 2024 Feb;116:303-316. doi: 10.1016/j.bbi.2023.12.034. Epub 2023 Dec 25. Brain Behav Immun. 2024. PMID: 38151165 Free PMC article. - Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis.
Wu A, Zhang J. Wu A, et al. J Neuroinflammation. 2023 Nov 27;20(1):283. doi: 10.1186/s12974-023-02964-x. J Neuroinflammation. 2023. PMID: 38012702 Free PMC article. Review. - Advances in the study of the molecular biological mechanisms of radiation-induced brain injury.
Gan C, Li W, Xu J, Pang L, Tang L, Yu S, Li A, Ge H, Huang R, Cheng H. Gan C, et al. Am J Cancer Res. 2023 Aug 15;13(8):3275-3299. eCollection 2023. Am J Cancer Res. 2023. PMID: 37693137 Free PMC article. Review. - Potential role between inflammatory cytokines and Tie-2 receptor levels and clinical symptoms in patients with first-episode schizophrenia.
Yan F, Meng X, Cheng X, Pei W, Chen Y, Chen L, Zheng M, Shi L, Zhu C, Zhang X. Yan F, et al. BMC Psychiatry. 2023 Jul 25;23(1):538. doi: 10.1186/s12888-023-04913-7. BMC Psychiatry. 2023. PMID: 37491201 Free PMC article.
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. - PubMed
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. - PubMed
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM07356/GM/NIGMS NIH HHS/United States
- R01 AG030149-05/AG/NIA NIH HHS/United States
- R01 AG030149/AG/NIA NIH HHS/United States
- R01 AG030149-03/AG/NIA NIH HHS/United States
- T32 GM007356-36/GM/NIGMS NIH HHS/United States
- AG030149/AG/NIA NIH HHS/United States
- T32 GM007356/GM/NIGMS NIH HHS/United States
- R01 AG030149-04/AG/NIA NIH HHS/United States
- T32 GM007356-35/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases