Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma - PubMed (original) (raw)
Clinical Trial
. 2011 Dec 1;118(23):6050-6.
doi: 10.1182/blood-2011-05-354449. Epub 2011 Oct 7.
Barbara Savoldo, Gianpietro Dotti, Martin Pule, Eric Yvon, G Doug Myers, Claudia Rossig, Heidi V Russell, Oumar Diouf, Enli Liu, Hao Liu, Meng-Fen Wu, Adrian P Gee, Zhuyong Mei, Cliona M Rooney, Helen E Heslop, Malcolm K Brenner
Affiliations
- PMID: 21984804
- PMCID: PMC3234664
- DOI: 10.1182/blood-2011-05-354449
Clinical Trial
Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma
Chrystal U Louis et al. Blood. 2011.
Abstract
We generated MHC-independent chimeric antigen receptors (CARs) directed to the GD2 antigen expressed by neuroblastoma tumor cells and treated patients with this disease. Two distinguishable forms of this CAR were expressed in EBV-specific cytotoxic T lymphocytes (EBV-CTLs) and activated T cells (ATCs). We have previously shown that EBV-CTLs expressing GD2-CARs (CAR-CTLs) circulated at higher levels than GD2-CAR ATCs (CAR-ATCs) early after infusion, but by 6 weeks, both subsets became low or undetectable. We now report the long-term clinical and immunologic consequences of infusions in 19 patients with high-risk neuroblastoma: 8 in remission at infusion and 11 with active disease. Three of 11 patients with active disease achieved complete remission, and persistence of either CAR-ATCs or CAR-CTLs beyond 6 weeks was associated with superior clinical outcome. We observed persistence for up to 192 weeks for CAR-ATCs and 96 weeks for CAR-CTLs, and duration of persistence was highly concordant with the percentage of CD4(+) cells and central memory cells (CD45RO(+)CD62L(+)) in the infused product. In conclusion, GD2-CAR T cells can induce complete tumor responses in patients with active neuroblastoma; these CAR T cells may have extended, low-level persistence in patients, and such persistence was associated with longer survival. This study is registered at www.clinialtrials.gov as #NCT00085930.
Figures
Figure 1
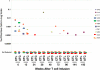
Composition of GD2–T-cell products. The cellular composition of GD2-CTL (A) and GD2-ATC (B) lines was analyzed using flow cytometry. Both products were polyclonal with a predominance of CD8+ T cells, followed by a smaller percentage of CD4+ cells and NK cells within each product line. Furthermore, as expected, GD2-CTL lines were terminally differentiated and composed of mostly effector memory cells (C), whereas GD2-ATCs (D) had a higher percentage of naive and central memory cells within each T-cell line.
Figure 2
First-generation GD2-CAR T cells can be detected in the peripheral blood of patients for a prolonged period of time. Real-time quantitative PCR was used to assess the presence and persistence of CAR–T cells. GD2-ATCs or GD2-CTLs could be detected, at or after 6 weeks after infusion, in the circulation of 11 of 19 (58%) patients. Although the levels of detection were low, transgenic signals could be identified at 96 weeks for CAR-CTLs and 192 weeks for CAR-ATCs after infusion. The estimate of frequency of CAR– T cells was obtained using standard curves of DNA from tumor cell lines transduced with the retroviral vectors encoding each CAR. Integrant analysis showed between 6 and 8 proviral integration sites per cell. Sensitivity assays in which transduced T cells were diluted with nontransduced T cells showed unequivocal detection ability when 1 transduced cell was diluted in 1000 to 6000 nontransduced cells, corresponding to 0.0001% to 0.002% of the tumor cell lines used for the standard curve.
Figure 3
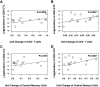
Prolonged detection of GD2–T cells within the peripheral blood of patients was highly concordant with the percentage of helper (CD4+ cells) and central memory cells (CD45RO+CD62L+) in the infused product. Using regression analysis, it was determined that each 1-unit increase in the number of CD4+ cells within the infused product was associated with a 2.28 increase in the log (duration) of CAR-CTL (A) and a 9.83 increase in the log (duration) of CAR-ATC (B). Further, each 1-unit increase in the percentage of central memory cells was associated with a 6.1 increase in the log (duration) of CAR-CTL (C) and a 6.63 increase in the log (duration) of CAR-ATC (D) in the T-cell product.
Figure 4
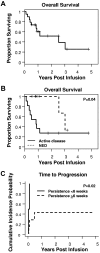
Detection of CAR–T cells for 6 weeks or more was associated with prolongation of TTP. (A) With a median follow-up of 329 days, the median OS was 931 days. (B) Patients with active disease at the time of infusion had a shorter OS compared with those with no evidence of disease. (C) In addition, for those with active disease at the time of infusion, the lack of CAR-ATCs or CAR-CTLs at or beyond 6 weeks was associated with a significantly shorter TTP.
Similar articles
- Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition.
Perna SK, Pagliara D, Mahendravada A, Liu H, Brenner MK, Savoldo B, Dotti G. Perna SK, et al. Clin Cancer Res. 2014 Jan 1;20(1):131-9. doi: 10.1158/1078-0432.CCR-13-1016. Epub 2013 Oct 4. Clin Cancer Res. 2014. PMID: 24097874 Free PMC article. - A novel anti-GD2/4-1BB chimeric antigen receptor triggers neuroblastoma cell killing.
Prapa M, Caldrer S, Spano C, Bestagno M, Golinelli G, Grisendi G, Petrachi T, Conte P, Horwitz EM, Campana D, Paolucci P, Dominici M. Prapa M, et al. Oncotarget. 2015 Sep 22;6(28):24884-94. doi: 10.18632/oncotarget.4670. Oncotarget. 2015. PMID: 26298772 Free PMC article. - High-Affinity GD2-Specific CAR T Cells Induce Fatal Encephalitis in a Preclinical Neuroblastoma Model.
Richman SA, Nunez-Cruz S, Moghimi B, Li LZ, Gershenson ZT, Mourelatos Z, Barrett DM, Grupp SA, Milone MC. Richman SA, et al. Cancer Immunol Res. 2018 Jan;6(1):36-46. doi: 10.1158/2326-6066.CIR-17-0211. Epub 2017 Nov 27. Cancer Immunol Res. 2018. PMID: 29180536 Free PMC article. - CAR T Cell Therapy for Neuroblastoma.
Richards RM, Sotillo E, Majzner RG. Richards RM, et al. Front Immunol. 2018 Oct 16;9:2380. doi: 10.3389/fimmu.2018.02380. eCollection 2018. Front Immunol. 2018. PMID: 30459759 Free PMC article. Review. - Advances in chimeric antigen receptor immunotherapy for neuroblastoma.
Heczey A, Louis CU. Heczey A, et al. Discov Med. 2013 Dec;16(90):287-94. Discov Med. 2013. PMID: 24333408 Free PMC article. Review.
Cited by
- Manufacture of tumor- and virus-specific T lymphocytes for adoptive cell therapies.
Wang X, Rivière I. Wang X, et al. Cancer Gene Ther. 2015 Mar;22(2):85-94. doi: 10.1038/cgt.2014.81. Epub 2015 Feb 27. Cancer Gene Ther. 2015. PMID: 25721207 Free PMC article. Review. - Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells.
Marin V, Cribioli E, Philip B, Tettamanti S, Pizzitola I, Biondi A, Biagi E, Pule M. Marin V, et al. Hum Gene Ther Methods. 2012 Dec;23(6):376-86. doi: 10.1089/hgtb.2012.050. Hum Gene Ther Methods. 2012. PMID: 23186165 Free PMC article. - New aspects of neuroblastoma treatment: ASPHO 2011 symposium review.
Zage PE, Louis CU, Cohn SL. Zage PE, et al. Pediatr Blood Cancer. 2012 Jul 1;58(7):1099-105. doi: 10.1002/pbc.24116. Epub 2012 Feb 29. Pediatr Blood Cancer. 2012. PMID: 22378620 Free PMC article. Review. - Acellular Wharton's Jelly, Potentials in T-Cell Subtypes Differentiation, Activation and Proliferation.
Talebi M, Nozad Charoudeh H, Movassaghpour Akbari AA, Baradaran B, Kazemi T. Talebi M, et al. Adv Pharm Bull. 2020 Sep;10(4):617-622. doi: 10.34172/apb.2020.074. Epub 2020 Aug 9. Adv Pharm Bull. 2020. PMID: 33072540 Free PMC article. - AKT Isoforms in the Immune Response in Cancer.
Ahmad Z, Somanath PR. Ahmad Z, et al. Curr Top Microbiol Immunol. 2022;436:349-366. doi: 10.1007/978-3-031-06566-8_15. Curr Top Microbiol Immunol. 2022. PMID: 36243852
References
- Raffaghello L, Prigione I, Airoldi I, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005;228(1):155–161. - PubMed
- Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. - PubMed
- Prigione I, Corrias MV, Airoldi I, et al. Immunogenicity of human neuroblastoma. Ann N Y Acad Sci. 2004;1028:69–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 RR000188/RR/NCRR NIH HHS/United States
- 5K12CA090433/CA/NCI NIH HHS/United States
- T32 HL092332/HL/NHLBI NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- T32HL092332/HL/NHLBI NIH HHS/United States
- RR00188/RR/NCRR NIH HHS/United States
- K12 CA090433/CA/NCI NIH HHS/United States
- P01 CA094237/CA/NCI NIH HHS/United States
- P01 CA94237/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials