The enlarged lysosomes in beige j cells result from decreased lysosome fission and not increased lysosome fusion - PubMed (original) (raw)
The enlarged lysosomes in beige j cells result from decreased lysosome fission and not increased lysosome fusion
Nina Durchfort et al. Traffic. 2012 Jan.
Abstract
Chediak-Higashi syndrome is an autosomal recessive disorder that affects vesicle morphology. The Chs1/Lyst protein is a member of the BEige And CHediak family of proteins. The absence of Chs1/Lyst gives rise to enlarged lysosomes. Lysosome size is regulated by a balance between vesicle fusion and fission and can be reversibly altered by acidifying the cytoplasm using Acetate Ringer's or by incubating with the drug vacuolin-1. We took advantage of these procedures to determine rates of lysosome fusion and fission in the presence or absence of Chs1/Lyst. Here, we show by microscopy, flow cytometry and in vitro fusion that the absence of the Chs1/Lyst protein does not increase the rate of lysosome fusion. Rather, our data indicate that loss of this protein decreases the rate of lysosome fission. We further show that overexpression of the Chs1/Lyst protein gives rise to a faster rate of lysosome fission. These results indicate that Chs1/Lyst regulates lysosome size by affecting fission.
© 2011 John Wiley & Sons A/S.
Conflict of interest statement
Competing Interests
The authors declare no competing financial interests.
Figures
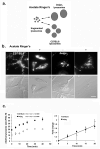
Figure 1. Acetate Ringer’s treatment results in fragmentation of lysosomes in wild type and beigej bone marrow-derived macrophages
a. Model for fragmented vacuole recovery in C57BL/6 versus beigej macrophages. Acetate Ringer’s causes lysosome fragmentation. Once Acetate Ringer’s is removed, cells regain their respective lysosome size with time. b. Bone marrow-derived macrophages from C57BL/6 or beigej mice were incubated with Alexa 488 dextran (mw 10,000) at 37°C overnight followed by a two h chase to allow all dextran to localize to lysosomes. Cells were incubated with Acetate Ringer’s for 20 min at 37°C, washed 3 times at 0°C. Epifluorescence images were captured using an Olympus BX51 upright microscope with a 60X 1.4NA objective and Pictureframer software (Olympus). c. Cells as in b were treated with Acetate Ringer’s to allow lysosomes to fragment and then placed back in normal growth media for the indicated times to allow lysosomes to refuse. Relative lysosome size during recovery was determined by flow cytometry (left panel) and the initial fold change in lysosome size over time plotted (right panel). Error bars represent the standard error of the mean. All experiments were performed a minimum of three times. Bar = 10 μm.
Figure 2. Vacuolin-1 increases lysosome size in C57BL/6 and beigej bone marrow-derived macrophages
Bone marrow-derived macrophages from C57BL/6 and beigej mice were grown on glass coverslips in macrophage growth medium. Cells were incubated with Alexa 594 dextran (mw 10,000) at 37°C overnight followed by a two h chase to allow all dextran to localize to lysosomes. Cells were incubated at 37°C for one h without or with 5 μM vacuolin-1 and images captured on an epifluorescence microscope. Vacuolin-1 was removed, cells incubated in growth media and at eight h and lysosome morphology analyzed by microscopy (n=6 with over 100 cells/cell type analyzed). Representative images are shown. Bar = 10 μm.
Figure 3. Vacuolin-1 increases lysosome size inmouse and human fibroblasts
Fibroblasts cell lines C57BL/6, beigej and YAC-complemented beigej as well as human control fibroblasts (HSFW11) and fibroblasts from a patient with Chediak Higashi syndrome (GM02075) were grown on glass coverslips in DMEM with 10% FBS. Cells were incubated with Alexa 594 dextran (mw 10,000) at 37°C overnight followed by a two h chase to allow all dextran to localize to lysosomes. Cells were incubated at 37°C for one h a without or b with 5 μM vacuolin-1 and lysosome morphology analyzed by microscopy (n=5 with over 100 cells/type examined). Bar = 10 μm.
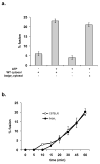
Figure 4. The absence of Chs1/Lyst delays lysosome recovery after vacuolin-1 treatment
a. Cells incubated with vacuolin-1 as in Fig. 3 were washed and placed in DMEM with 10% FBS for four h and lysosome morphology examined by fluorescence microscopy. b. and c. Cells treated as in Fig. 3 were placed in growth medium, images captured at defined times and cell recovery determined by contrasting several fields (2–10 fields) of untreated cells to several fields (2–10 fields) of treated cells without recovery and then counting the number of cells at each recovery time that were more like untreated or treated cells. The data are expressed as the percentage of cells showing lysosome recovery. The experiments were performed three times and the data represent the average of 10–20 fields of cells with 2–10 cells per field (40–100 cells/cell type). d. Cells were incubated with or without 100 μg/ml cycloheximide during vacuolin-1 recovery and cell recovery determined at eight h by contrasting untreated cells to treated cells as in b and c. Bar = 10 μm.
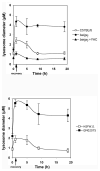
Figure 5. Measurement of lysosome size during vacuolin-1 recovery
Fibroblasts were incubated with Alexa 594-dextran overnight at 37°C. Cells were washed and incubated in growth medium for an additional two h. Cells were treated with or without vacuolin-1 for one h at 37°C followed by incubation in growth medium for indicated times. Lysosome morphology was examined by epifluorescence microscopy and images analyzed using Image J software measuring lysosome size (diameter μm) from three separate experiments. The data represent the average of 10–20 fields with two to 10 cells per field. Over 200 vesicles per cell type were measured at each time point. Error bars represent the standard error of the mean.
Figure 6. In vitro lysosome fusion is not affected by the absence Lyst
a. Alveolar macrophages were isolated as described previously (7). Macrophages were incubated with either 0.5 mg/ml biotinylated-horseradish peroxidase or avidin at 37°C for 60 min. Cells were washed extensively and chased for an additional two h. Cells were washed, homogenized and lysosomes isolated as previously described (7). Lysosomes (1–2 mg/ml) were combined at 4°C in the presence of excess biotinylated-insulin with or without an ATP-regenerating system and with or without cytosol isolated from wild type C57BL/6 liver or beigej liver (2–4 mg/ml). Samples were shifted to 37°C for 60 min, fusion stopped by placing samples at 4°C and fusion assayed as previously described (7). In vitro fusion was expressed as the percentage of maximum avidin-b-HRP complex formation. b. Samples as in 5a where shifted to 37°C for time to measure the kinetics of in vitro fusion in the presence or absence of Chs1/Lyst. All experiments were performed in triplicate and error bars represent the standard error of the mean.
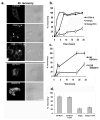
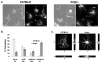
Figure 7. Bone marrow-derived macrophage lysosomes in beigej show increased tubular morphology
Wild type and beigej bone marrow-derived macrophages were cultured, plated on glass coverslips and labeled as described in Fig. 2. a. Images were captured on an FV1000 Olympus confocal microscope. Bar = 10 μm. b. The number of cells (greater than 200 cells each) showing vesicular or tubular lysosomes was quantified by four different individuals using the same range and same three planes of focus to determine no tubular lysosomes (N), primarily vesicles with a few tubular lysosomes (Mi), approximately equivalent numbers of vesicular or tubular lysosomes medium (Me) or predominantly tubular lysosomes with few vesicular lysosomes as extreme (E) and the average plus and minus the standard error of the mean of three separate experiments used to determine the percent tubulation. c. Confocal images of extreme tubulation in C57BL/6 and beigej bone marrow-derived macrophages showing xy, xz and yz projections. Bar = 5 μm.
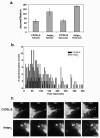
Figure 8. Wild type lysosomes show increased fission rates compared to beigej lysosomes
a. Wild type and beigej macrophages loaded with Alexa 594 dextran as in Fig. 7 were imaged on a Nikon automated Metamorph microscope capturing six different fields with five-to-ten cells per field (n=2 separate experiments). Images were captured every 5 sec for up to ten min. Movies were generated and image analysis performed with NIH Image J software measuring time elapsed to vesicle/tubule fission. Between 50–150 vesiculation events were quantified/cell type. The data are expressed as seconds/fission event in either tubular or vesicular lysosomes. b. The combined data from a are plotted as a histogram with the data expressed as the number of vesiculation events (Y axis) per second (X axis). c. Shown are representative snapshots of lysosome fission in C57BL/6 and beigej over 210 sec. Arrowheads identify vesicle formation and arrows identify incomplete fission events. Bar = 5 μm.
Similar articles
- LYST affects lysosome size and quantity, but not trafficking or degradation through autophagy or endocytosis.
Holland P, Torgersen ML, Sandvig K, Simonsen A. Holland P, et al. Traffic. 2014 Dec;15(12):1390-405. doi: 10.1111/tra.12227. Epub 2014 Oct 8. Traffic. 2014. PMID: 25216107 - Chediak-Higashi Syndrome: a rare disorder of lysosomes and lysosome related organelles.
Shiflett SL, Kaplan J, Ward DM. Shiflett SL, et al. Pigment Cell Res. 2002 Aug;15(4):251-7. doi: 10.1034/j.1600-0749.2002.02038.x. Pigment Cell Res. 2002. PMID: 12100490 Review. - Chediak-Higashi syndrome.
Kaplan J, De Domenico I, Ward DM. Kaplan J, et al. Curr Opin Hematol. 2008 Jan;15(1):22-9. doi: 10.1097/MOH.0b013e3282f2bcce. Curr Opin Hematol. 2008. PMID: 18043242 Review. - Dictyostelium LvsB has a regulatory role in endosomal vesicle fusion.
Falkenstein K, De Lozanne A. Falkenstein K, et al. J Cell Sci. 2014 Oct 15;127(Pt 20):4356-67. doi: 10.1242/jcs.138123. Epub 2014 Aug 1. J Cell Sci. 2014. PMID: 25086066 Free PMC article. - LYST deficiency impairs autophagic lysosome reformation in neurons and alters lysosome number and size.
Serra-Vinardell J, Sandler MB, De Pace R, Manzella-Lapeira J, Cougnoux A, Keyvanfar K, Introne WJ, Brzostowski JA, Ward ME, Gahl WA, Sharma P, Malicdan MCV. Serra-Vinardell J, et al. Cell Mol Life Sci. 2023 Jan 28;80(2):53. doi: 10.1007/s00018-023-04695-x. Cell Mol Life Sci. 2023. PMID: 36707427 Free PMC article.
Cited by
- Mechanisms of protein delivery to melanosomes in pigment cells.
Sitaram A, Marks MS. Sitaram A, et al. Physiology (Bethesda). 2012 Apr;27(2):85-99. doi: 10.1152/physiol.00043.2011. Physiology (Bethesda). 2012. PMID: 22505665 Free PMC article. Review. - Familial hemophagocytic lymphohistiocytosis: when rare diseases shed light on immune system functioning.
Sieni E, Cetica V, Hackmann Y, Coniglio ML, Da Ros M, Ciambotti B, Pende D, Griffiths G, Aricò M. Sieni E, et al. Front Immunol. 2014 Apr 16;5:167. doi: 10.3389/fimmu.2014.00167. eCollection 2014. Front Immunol. 2014. PMID: 24795715 Free PMC article. Review. - Neurologic involvement in patients with atypical Chediak-Higashi disease.
Introne WJ, Westbroek W, Groden CA, Bhambhani V, Golas GA, Baker EH, Lehky TJ, Snow J, Ziegler SG, Malicdan MC, Adams DR, Dorward HM, Hess RA, Huizing M, Gahl WA, Toro C. Introne WJ, et al. Neurology. 2017 Feb 14;88(7):e57-e65. doi: 10.1212/WNL.0000000000003622. Neurology. 2017. PMID: 28193763 Free PMC article. - Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress.
Mackey E, Ayyadurai S, Pohl CS, D' Costa S, Li Y, Moeser AJ. Mackey E, et al. Biol Sex Differ. 2016 Nov 22;7:60. doi: 10.1186/s13293-016-0113-7. eCollection 2016. Biol Sex Differ. 2016. PMID: 27895892 Free PMC article. - The Rab7 effector WDR91 promotes autophagy-lysosome degradation in neurons by regulating lysosome fusion.
Xing R, Zhou H, Jian Y, Li L, Wang M, Liu N, Yin Q, Liang Z, Guo W, Yang C. Xing R, et al. J Cell Biol. 2021 Aug 2;220(8):e202007061. doi: 10.1083/jcb.202007061. Epub 2021 May 24. J Cell Biol. 2021. PMID: 34028500 Free PMC article.
References
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8(8):622–632. - PubMed
- Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr Biol. 2005;15(4):360–365. - PubMed
- Murphy RF. Maturation models for endosome and lysosome biogenesis. Trends Cell Biol. 1991;1(4):77–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources