Thyroid iodide efflux: a team effort? - PubMed (original) (raw)
Review
Thyroid iodide efflux: a team effort?
Peying Fong. J Physiol. 2011.
Abstract
The thyroid hormones thyroxine (T(4)) and triiodothyronine (T(3)) play key roles in regulating development, growth and metabolism in pre- and postnatal life. Iodide (I(-)) is an essential component of the thyroid hormones and is accumulated avidly by the thyroid gland. The rarity of elemental iodine and I(-) in the environment challenges the thyroid to orchestrate a remarkable series of transport processes that ultimately ensure sufficient levels for hormone synthesis. In addition to actively extracting circulating I(-), thyroid follicular epithelial cells must also translocate I(-) into a central intrafollicular compartment, where thyroglobulin is iodinated to form the protein precursor to T(4) and T(3). In the last decade, several bodies of evidence render questionable the notion that I(-) exits thyrocytes solely via the Cl(-)/I(-) exchanger Pendrin (SLC26A4), therefore necessitating reconsideration of several other candidate I(-) conduits: the Cl(-)/H(+) antiporter, CLC-5, the cystic fibrosis transmembrane conductance regulator (CFTR) and the sodium monocarboxylic acid transporter (SMCT1).
Figures
Figure 1. Organization of thyroid follicular epithelium and summary of key processes in hormone synthesis
A, haematoxylin and eosin stained section of neonatal pig thyroid tissue showing follicles consisting of epithelial cells and colloid-filled lumina. Scale bar = 50 μm. B, schematic depiction of thyroid follicle showing polarized orientation of epithelial cells (A, apical; BL, basolateral). Arrows indicate directional movements of thyroglobulin (Tg) and I−, uptake of iodinated Tg (Tg*) pro-hormone and release of T3 and T4. Circulating I− levels in blood range between 10 and 100 μ
m
(Kogai et al. 2006; Shcheynikov et al. 2008). Due to rapid intrafollicular Tg* formation, the colloid can be regarded as essentially devoid of free I−. C, basic steps involved in thyroid hormone formation are shown. Left, TSH binds its receptor and promotes thyroid hormone production. Circulating I− is taken up at the basolateral membrane via the sodium-iodide symporter, NIS (Dai et al. 1996). Intracellular I− accumulates and moves across the apical membrane by a process believed at least partially to be mediated by Pendrin, thereby providing this essential element for hormone synthesis. Middle, Tg is produced by the thyroid epithelial cells, processed along the synthetic pathway and released into the follicular lumen. Top, H2O2, required for the coupling of I− and Tg, is generated by DUOX2 at the apical membrane. Reaction of these components is catalysed by TPO; I− is coupled to many tyrosine residues within Tg, of which only a select number are hormonogenic. Tyrosine residues can be either mono- or di-iodinated. TPO also facilitates coupling of these residues to form precursor to T3 (mono- + di-iodinated) and T4 (2 × di-iodinated). Right, outline of endocytosis and processing of the pro-hormone. Some Tg* is proteolysed at the apical surface by externalized cathepsins prior to uptake (Brix et al. 1996). Internalized Tg* is proteolysed by cathepsins within lysosomes (Dunn et al. 1991; Dunn & Dunn, 2001). T3 and T4 exit basolaterally in a process at least partially mediated by MCT8, a monocarboxylic acid transporter of the SLC16 family (Di Cosmo et al. 2010). Un-utilized iodotyrosines are further degraded by iodotyrosine dehalogenases and recycled.
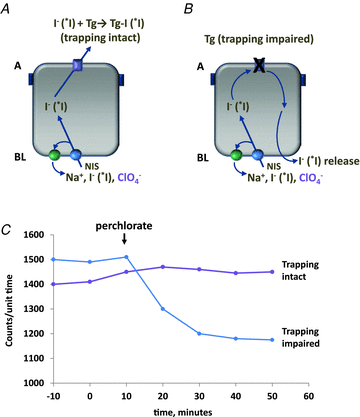
Figure 2. Outline of the perchlorate discharge test
Subjects are infused with radioiodide tracer (*I) prior to perchlorate challenge_. A_, intact trapping depends on efficient exit of I− (*I) from the cell and into the follicle lumen. On challenge with perchlorate, levels generally remain steady; any decline is <10% below baseline. B, impaired exit of I− (*I) shown in this panel. Trapping cannot proceed without transport of I− (*I) into the follicular lumen. Perchlorate competes for NIS-mediated uptake; untrapped intracellular I− (*I) is released basolaterally into the circulation, resulting in a decrease in thyroid counts. C, this idealized plot illustrates time courses of radioiodide counts measured over the thyroid after perchlorate administration (arrow) in the case of either intact or impaired trapping.
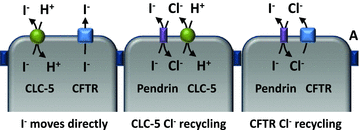
Figure 3. Models showing how CLC-5 and CFTR might directly facilitate I− efflux, as well as indirectly by coupling with Pendrin
Left, direct I− transport via either CLC-5 and/or CFTR. Middle, efflux of Cl− through CLC-5 provides a counter-ion for Pendrin-mediated I− efflux. Note that stoichiometry of CLC-5 is 2 Cl− to 1 H+; only one Cl− is shown for simplicity. Right, similarly, CFTR-mediated Cl− efflux also can furnish a counter-ion for driving Pendrin exchange activity.
Similar articles
- The loss of the chloride channel, ClC-5, delays apical iodide efflux and induces a euthyroid goiter in the mouse thyroid gland.
van den Hove MF, Croizet-Berger K, Jouret F, Guggino SE, Guggino WB, Devuyst O, Courtoy PJ. van den Hove MF, et al. Endocrinology. 2006 Mar;147(3):1287-96. doi: 10.1210/en.2005-1149. Epub 2005 Nov 23. Endocrinology. 2006. PMID: 16306076 - Controversies concerning the role of pendrin as an apical iodide transporter in thyroid follicular cells.
Bizhanova A, Kopp P. Bizhanova A, et al. Cell Physiol Biochem. 2011;28(3):485-90. doi: 10.1159/000335103. Epub 2011 Nov 18. Cell Physiol Biochem. 2011. PMID: 22116361 Review. - Pendrin and anoctamin as mediators of apical iodide efflux in thyroid cells.
Silveira JC, Kopp PA. Silveira JC, et al. Curr Opin Endocrinol Diabetes Obes. 2015 Oct;22(5):374-80. doi: 10.1097/MED.0000000000000188. Curr Opin Endocrinol Diabetes Obes. 2015. PMID: 26313899 Review. - Genetics and phenomics of Pendred syndrome.
Bizhanova A, Kopp P. Bizhanova A, et al. Mol Cell Endocrinol. 2010 Jun 30;322(1-2):83-90. doi: 10.1016/j.mce.2010.03.006. Epub 2010 Mar 15. Mol Cell Endocrinol. 2010. PMID: 20298745 Review. - Pendred syndrome and iodide transport in the thyroid.
Kopp P, Pesce L, Solis-S JC. Kopp P, et al. Trends Endocrinol Metab. 2008 Sep;19(7):260-8. doi: 10.1016/j.tem.2008.07.001. Epub 2008 Aug 7. Trends Endocrinol Metab. 2008. PMID: 18692402 Review.
Cited by
- The Role of Inositol in Thyroid Physiology and in Subclinical Hypothyroidism Management.
Benvenga S, Nordio M, Laganà AS, Unfer V. Benvenga S, et al. Front Endocrinol (Lausanne). 2021 May 10;12:662582. doi: 10.3389/fendo.2021.662582. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34040582 Free PMC article. Review. - Acute inhibition of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel by thyroid hormones involves multiple mechanisms.
Cai Z, Li H, Chen JH, Sheppard DN. Cai Z, et al. Am J Physiol Cell Physiol. 2013 Oct 15;305(8):C817-28. doi: 10.1152/ajpcell.00052.2013. Epub 2013 Jun 19. Am J Physiol Cell Physiol. 2013. PMID: 23784545 Free PMC article. - Iodide Binding in Sodium-Coupled Cotransporters.
Vergara-Jaque A, Fong P, Comer J. Vergara-Jaque A, et al. J Chem Inf Model. 2017 Dec 26;57(12):3043-3055. doi: 10.1021/acs.jcim.7b00521. Epub 2017 Dec 4. J Chem Inf Model. 2017. PMID: 29131623 Free PMC article. - Histopathological Features of Pendred Syndrome Thyroids Align with Differences in the Expression of Thyroid-Specific Markers, Apical Iodide Transporters, and Ciliogenesis Process.
Vázquez-Román V, Cameselle-Teijeiro JM, Fernández-Santos JM, Ríos-Moreno MJ, Loidi L, Ortiz T, Martín-Lacave I. Vázquez-Román V, et al. Endocr Pathol. 2022 Dec;33(4):484-493. doi: 10.1007/s12022-022-09732-2. Epub 2022 Oct 15. Endocr Pathol. 2022. PMID: 36242759 Free PMC article. - The SLC26 gene family of anion transporters and channels.
Alper SL, Sharma AK. Alper SL, et al. Mol Aspects Med. 2013 Apr-Jun;34(2-3):494-515. doi: 10.1016/j.mam.2012.07.009. Mol Aspects Med. 2013. PMID: 23506885 Free PMC article. Review.
References
- Aravind L, Koonin EV. The STAS domain – a link between anion transporters and antisigma-factor antagonists. Curr Biol. 2000;10:R53–R55. - PubMed
- Armstrong J, Matainaho T, Cragoe EJ, Jr, Huxham GJ, Bourke JR, Manley SW. Bidirectional ion transport in thyroid: secretion of anions by monolayer cultures that absorb sodium. Am J Physiol Endocrinol Metab. 1992a;262:E40–E45. - PubMed
- Armstrong JW, Cragoe EJ, Jr, Bourke JR, Huxham GJ, Manley SW. Chloride conductance of apical membrane in cultured porcine thyroid cells activated by cyclic AMP. Mol Cell Endocrinol. 1992b;88:105–110. - PubMed
- Bagchi N, Fawcett DM. Role of sodium ion in active transport of iodide by cultured thyroid cells. Biochim Biophys Acta. 1973;318:235–251. - PubMed
- Bourke JR, Sand O, Abel KC, Huxham GJ, Manley SW. Chloride channels in the apical membrane of thyroid epithelial cells are regulated by cyclic AMP. J Endocrinol. 1995;147:441–448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous