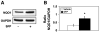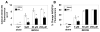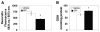Sulforaphane inhibits mitochondrial permeability transition and oxidative stress - PubMed (original) (raw)
Sulforaphane inhibits mitochondrial permeability transition and oxidative stress
Tiffany Greco et al. Free Radic Biol Med. 2011.
Abstract
Exposure of mitochondria to oxidative stress and elevated Ca(2+) promotes opening of the mitochondrial permeability transition pore (PTP), resulting in membrane depolarization, uncoupling of oxidative phosphorylation, and potentially cell death. This study tested the hypothesis that treatment of rats with sulforaphane (SFP), an activator of the Nrf2 pathway of antioxidant gene expression, increases the resistance of liver mitochondria to redox-regulated PTP opening and elevates mitochondrial levels of antioxidants. Rats were injected with SFP or drug vehicle and liver mitochondria were isolated 40h later. Respiring mitochondria actively accumulated added Ca(2+), which was then released through PTP opening induced by agents that either cause an oxidized shift in the mitochondrial redox state or directly oxidize protein thiol groups. SFP treatment of rats inhibited the rate of pro-oxidant-induced mitochondrial Ca(2+) release and increased expression of the glutathione peroxidase/reductase system, thioredoxin, and malic enzyme. These results are the first to demonstrate that SFP treatment of animals increases liver mitochondrial antioxidant defenses and inhibits redox-sensitive PTP opening. This novel form of preconditioning could protect against a variety of pathologies that include oxidative stress and mitochondrial dysfunction in their etiologies.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Fig. 1
NAD(P)H quinone oxidoreductase 1 (NQO1) immunoreactivity in liver homogenates from vehicle- and sulforaphane (SFP) treated rats. A. Representative immunoblots for NQO1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). B. Densitometric ratios for NQO1/VDAC for n = 4–6 animals per group. * p<0.05, t=2.81
Fig. 2
Peroxide-induced permeability pore transition (PTP) opening and pyridine nucleotide oxidation in rat liver mitochondria. A. Representative tracings of respiration-dependent mitochondrial Ca2+ uptake and subsequent PTP-dependent release. Extramitochondrial free Ca2+ was monitored with the Calcium Green 5N fluorescent dye in medium containing succinate as a respiratory substrate and the respiratory chain complex I inhibitor, rotenone. At time 0 mitochondria (0.5 mg/ml) from vehicle-treated or sulforaphane (SFP)-treated rats were loaded with 25 μM calcium, which is a level that was retained almost completely during the duration of the measurements (Line 1 & 4). At 250 sec, tert-butyl hydroperoxide (_t_BOOH) was added at either 5 μM (Line 2) or 250 μM (Line 3), resulting in an almost immediate increase in calcium green 5N fluorescence, representing mitochondrial Ca2+ release. Ca2+ efflux from control mitochondria was completely inhibited by the presence of 1 μM cyclosporin A (CsA) (Line 7) and was substantially greater than that observed with mitochondria from sulforaphane-treated rats (Lines 5 & 6). At 450 sec, ionomycin, a non-specific calcium ionophore, was added to completely release the small, residual mitochondrial Ca2+ (Line 3). B. Representative tracings of NAD(P)H autofluorescence in the absence and presence of _t_BOOH. Pyridine nucleotide redox state for mitochondria from either vehicle- or sulforaphane-treated rats remained highly reduced and stable after the addition of Ca2+ alone (Lines 1 & 4). The addition of 5 μM (Line 2) or 250 μM (Line 3) _t_BOOH caused immediate and complete oxidation of NAD(P)H within vehicle control mitochondria. Treatment of rats with SFP inhibited NAD(P)H oxidation at 5 μM _t_BOOH (Line 5), but did not inhibit oxidation at 250 μM _t_BOOH (Line 6).
Fig. 3
Comparison of peroxide induced Ca2+ release rates and pyridine nucleotide oxidation for mitochondria from sulforaphane treated and vehicle treated rats (n = 7/group). A. Initial rates of rise in Calcium Green 5N fluorescence after addition of 0, 5, 50, and 250 μM _t_BOOH were all significantly slower for mitochondria from sulforaphane-treated rats. * p<0.05, t=2.16–4.91. Extent to which NAD(P)H autofluorescence decreased after additions of different concentrations of _t_BOOH was significantly less at 5 μM _t_BOOH. * p<0.05, t=0.82–2.20.
Fig. 4
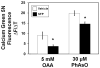
Inhibition of oxaloacetate- or phenylarsine oxide-induced Ca2+ release by rat liver mitochondria (n=4/group). A. Initial rates of rise in Calcium Green 5N fluorescence after the addition of 5 mM oxaloacetate (OAA) or B. 30 μM phenylarsine oxide (PhAsO) were significantly slower for sulforaphane-treated animals. * p<0.05, t=2.77–2.83.
Fig. 5
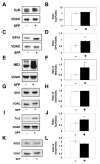
Effects of sulforaphane treatment on immunoreactive levels of mitochondrial proteins related to regulation of permeability transition pore opening (n=6–7/group). Representative immunoblots and densitometry of A, B. cyclophilin D (CyD)/voltage dependent anion channel (VDAC), t=1.14, C, D. glutathione peroxidase 1 (GPX1)/VDAC, t=2.47, E, F. malic enzyme 3 (ME3)/VDAC, t=2.25, G, H. isocitrate dehydrogenase 2 (IDH2)/VDAC, t=1.19, I, J. thioredoxin 2 (Trx2)/VDAC, t=4.05, K, L. superoxide dismutase 2 (SOD2)/VDAC, 0.52. * p<0.05
Fig. 6
Effects of sulforaphane treatment on mitochondrial peroxidase activity and glutathione content (n=7–10/group). A. Liver mitochondria were incubated with 1 mM _t_BOOH for one minute. An aliquot was added to a cuvette containing horseradish peroxidase (HRP) and amplex red. Any residual _t_BOOH not reduced to H2O reacts with HRP to oxidize amplex red into its fluorescent product, rezorufin. * p<0.05, t=2.39. B. Comparision of the content of reduced glutathione in liver mitochondria from either vehicle or sulforaphane treated rats. * p<0.05, t=2.93.
Similar articles
- Brain mitochondria from rats treated with sulforaphane are resistant to redox-regulated permeability transition.
Greco T, Fiskum G. Greco T, et al. J Bioenerg Biomembr. 2010 Dec;42(6):491-7. doi: 10.1007/s10863-010-9312-9. J Bioenerg Biomembr. 2010. PMID: 21061051 Free PMC article. - Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent of the classical mitochondrial permeability transition pore opening.
Puntel RL, Roos DH, Folmer V, Nogueira CW, Galina A, Aschner M, Rocha JB. Puntel RL, et al. Toxicol Sci. 2010 Sep;117(1):133-43. doi: 10.1093/toxsci/kfq185. Epub 2010 Jun 23. Toxicol Sci. 2010. PMID: 20573786 Free PMC article. - Mitochondrial calcium transport and the redox nature of the calcium-induced membrane permeability transition.
Vercesi AE, Castilho RF, Kowaltowski AJ, de Oliveira HCF, de Souza-Pinto NC, Figueira TR, Busanello ENB. Vercesi AE, et al. Free Radic Biol Med. 2018 Dec;129:1-24. doi: 10.1016/j.freeradbiomed.2018.08.034. Epub 2018 Aug 31. Free Radic Biol Med. 2018. PMID: 30172747 Review. - Influence of aging on membrane permeability transition in brain mitochondria.
Toman J, Fiskum G. Toman J, et al. J Bioenerg Biomembr. 2011 Feb;43(1):3-10. doi: 10.1007/s10863-011-9337-8. J Bioenerg Biomembr. 2011. PMID: 21311961 Free PMC article. Review.
Cited by
- Sulforaphane attenuates gentamicin-induced nephrotoxicity: role of mitochondrial protection.
Negrette-Guzmán M, Huerta-Yepez S, Medina-Campos ON, Zatarain-Barrón ZL, Hernández-Pando R, Torres I, Tapia E, Pedraza-Chaverri J. Negrette-Guzmán M, et al. Evid Based Complement Alternat Med. 2013;2013:135314. doi: 10.1155/2013/135314. Epub 2013 Apr 4. Evid Based Complement Alternat Med. 2013. PMID: 23662110 Free PMC article. - Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration.
Holmström KM, Baird L, Zhang Y, Hargreaves I, Chalasani A, Land JM, Stanyer L, Yamamoto M, Dinkova-Kostova AT, Abramov AY. Holmström KM, et al. Biol Open. 2013 Jun 20;2(8):761-70. doi: 10.1242/bio.20134853. eCollection 2013 Aug 15. Biol Open. 2013. PMID: 23951401 Free PMC article. - Nrf2, the Master Regulator of Anti-Oxidative Responses.
Vomund S, Schäfer A, Parnham MJ, Brüne B, von Knethen A. Vomund S, et al. Int J Mol Sci. 2017 Dec 20;18(12):2772. doi: 10.3390/ijms18122772. Int J Mol Sci. 2017. PMID: 29261130 Free PMC article. Review. - Genetic activation of Nrf2 protects against fasting-induced oxidative stress in livers of mice.
Zhang YK, Wu KC, Klaassen CD. Zhang YK, et al. PLoS One. 2013;8(3):e59122. doi: 10.1371/journal.pone.0059122. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527105 Free PMC article. - Epigenetic linkage of aging, cancer and nutrition.
Daniel M, Tollefsbol TO. Daniel M, et al. J Exp Biol. 2015 Jan 1;218(Pt 1):59-70. doi: 10.1242/jeb.107110. J Exp Biol. 2015. PMID: 25568452 Free PMC article. Review.
References
- Bianchi G, Marchesini G, Fabbri A, Ronchi M, Chianese R, Grossi G. Lipoperoxide plasma levels in patients with liver cirrhosis. Hepatogastroenterology. 1997;44:784–788. - PubMed
- Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, Yoshikawa T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem Biophys Res Commun. 1998;247:166–170. - PubMed
- Packer MA, Murphy MP. Peroxynitrite causes calcium efflux from mitochondria which is prevented by Cyclosporin A. FEBS Lett. 1994;345:237–240. - PubMed
- Moore M, Thor H, Moore G, Nelson S, Moldeus P, Orrenius S. The toxicity of acetaminophen and N-acetyl-p-benzoquinone imine in isolated hepatocytes is associated with thiol depletion and increased cytosolic Ca2+ J Biol Chem. 1985;260:13035–13040. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous