PEGylated Adenoviruses: From Mice to Monkeys - PubMed (original) (raw)
PEGylated Adenoviruses: From Mice to Monkeys
Piyanuch Wonganan et al. Viruses. 2010 Feb.
Abstract
Covalent modification with polyethylene glycol (PEG), a non-toxic polymer used in food, cosmetic and pharmaceutical preparations for over 60 years, can profoundly influence the pharmacokinetic, pharmacologic and toxciologic profile of protein and peptide-based therapeutics. This review summarizes the history of PEGylation and PEG chemistry and highlights the value of this technology in the context of the design and development of recombinant viruses for gene transfer, vaccination and diagnostic purposes. Specific emphasis is placed on the application of this technology to the adenovirus, the most potent viral vector with the most highly characterized toxicity profile to date, in several animal models.
Keywords: PEGylation; adenovirus; gene therapy; immune response; non-viral vectors; pharmacokinetics; targeting; tolerance; toxicity; vaccine.
Figures
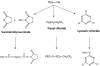
Figure 1.
Representative Types of PEG for Protein and Peptide Modification. (A) The core molecule, linear poly(ethylene) glycol, a diol, with two free hydroxyl groups; (B) Monomethoxy poly(ethylene) glycol (mPEG). The single hydroxyl group is the site of attachment for a variety of reactive groups suitable for conjugation to nucleophilic functional groups on proteins such as lysine; (C) A branched PEG molecule in which two linear mPEGs activated with either succinimidyl carbonate or benzotriazole are linked to the ∝- and ɛ-amino groups of lysine. These molecules offer the advantage of adding two PEG molecules at each attachment site, affording broader protection from proteolysis and the immune response without reducing bioactivity; (D) Multi-arm PEG. These compounds, generally prepared with hexaglycerine at the core, offer multiple hydroxyl groups for attachment of many copies of the same or several different reactive groups for protein and peptide conjugation; (E) Forked shaped PEG. Fork-shaped PEGs provide multiple reactive groups in close proximity at one or both ends (Panel F) of the PEG chain, where X represents functional groups.
Figure 1.
Representative Types of PEG for Protein and Peptide Modification. (A) The core molecule, linear poly(ethylene) glycol, a diol, with two free hydroxyl groups; (B) Monomethoxy poly(ethylene) glycol (mPEG). The single hydroxyl group is the site of attachment for a variety of reactive groups suitable for conjugation to nucleophilic functional groups on proteins such as lysine; (C) A branched PEG molecule in which two linear mPEGs activated with either succinimidyl carbonate or benzotriazole are linked to the ∝- and ɛ-amino groups of lysine. These molecules offer the advantage of adding two PEG molecules at each attachment site, affording broader protection from proteolysis and the immune response without reducing bioactivity; (D) Multi-arm PEG. These compounds, generally prepared with hexaglycerine at the core, offer multiple hydroxyl groups for attachment of many copies of the same or several different reactive groups for protein and peptide conjugation; (E) Forked shaped PEG. Fork-shaped PEGs provide multiple reactive groups in close proximity at one or both ends (Panel F) of the PEG chain, where X represents functional groups.
Figure 2.
General Schematic for the Synthesis of Activated PEG Molecules.
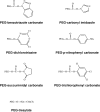
Figure 3.
First Generation PEG Derivatives.
Figure 4.
Second Generation PEG Derivatives. These PEG derivatives have been developed for specific attachment to cysteine residues on proteins and peptides. (A) PEG-maleimide is able to conjugate to free thiols under acidic (pH 6–7) conditions, however, this compound is not stable in water and the ring is susceptible to opening or addition of water across the double bond. (B) PEG-orthopyridyl disulfide reacts specifically with sulfhydryl groups under both acidic and basic conditions (pH 3–10) to form a disulfide bond with proteins which are stable except in a reducing environment when the linkage is converted to a thiol. (C) PEG-iodoacetamide, reacts slowly with thiol residues to form stable thioether bonds in mildly basic media. Use of this compound is advantageous in the context that, by strong acid hydrolysis, modification of a protein with this compound gives rise to a stable cysteine conjugate, carboxymethylcysteine that can be identified and quantified by standard amino acid sequencing techniques to verify the degree of modification of the parent protein. It is also important to note that any reaction employing this polymer should be performed under dark conditions in order to prevent the production of free iodine that may react with other amino acids such as tyrosine. (D) PEG-vinyl sulfone reacts slowly with thiols to form a stable thioester linkage to proteins under slightly basic conditions (pH 7–8). This process will proceed at a faster rate if the pH is increased. However, under these conditions, PEG-vinyl sulfone may also react with free lysines. Use of any of these compounds is dictated by protein solubility/stability under reaction conditions defined by the PEGylation chemistry, availability/accessibility of cysteine residues on the protein surface, desired speed of reaction and availability of methodology for characterization of protein conjugates.
Figure 5.
The Building Blocks of a PEGylated Therapeutic. Once a therapeutic molecule amenable to PEGylation is identified, the appropriate PEG chemistry must be selected. Properties such as size, symmetry, and bifunctionality must be considered and adopted according to the desired application. The linker used for covalent attachment of PEG must also be evaluated with respect to the strength of the bond created as well as its affinity for certain residues on the bioactive molecule. Receptor-specific peptides, proteins and molecular sensors have been tethered to PEGylated therapeutics for cellular and tissue specific targeting.
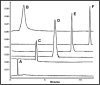
Figure 6.
PEGylation Significantly Alters the Surface Charge of the Adenovirus Over Time. Representative Capillary Electropherograms of (A) Monomethoxypoly(ethylene) glycol tresylate (TMPEG, 10 mg/ml) alone and adenovirus that underwent conjugation for (B) 24 hours (100% coverage as determined by a fluorescamine assay) (C) 4 hours (90% coverage) (D) 2 hours (70% coverage) (E) 1 hour (50% coverage) and (F) Unmodified Virus. As seen from the figure, capillary electrophoresis can be used to (a) confirm that free PEG has been adequately removed from a preparation, (b) assess the degree of modification of virus capsids and (c) assess the homogeneity of a preparation (i.e., all capsids are modified to the same degree). Data included in the figure was generated by diluting samples 1:2 with sample buffer (20 mM sodium phosphate, pH, 7.0, 5 mM NaCl). Capillary length was 34 cm. Virus was detected at 214 nm. The Y axis represents absorbance units and the X axis minutes until a preparation eluted from the capillary.
Figure 7.
PEGylation Dampens Peak Intensity of Adenovirus Capsid Proteins as Determined by Reverse Phase HPLC. RP-HPLC Chromatograms Showing Peaks of (A) Free PEG; (B) PEGylated adenovirus (50% modification as determined by CE and fluorescamine assays); (C) Unmodified Adenovirus and (D) PEGylated adenovirus (100% modification). Viral proteins were separated on a Jupiter column (250 × 4 mm) packed with a 5 μm diameter, 300 Å pore size C4 resin (Phenomenex) and a pre-column filter (0.5 μm, Phenomenex) at 45 °C. A 145 minute gradient of 0.1% trifluroacetic acid (TFA) in water (Solution A) and 0.1% TFA in acetonitrile (Solution B) was used at a flow rate of 1 ml/min and absorbance measured at 215 nm. Reduction of the peak height of the penton protein (Peak 2) reflects the degree of modification of the virus capsid as determined by fluorescamine and biotin ELISA assays (see table for data summary). This method can also be used to monitor the PEGylation process and confirm results obtained from analysis by capillary electrophoresis (Figure 6). It also verifies that free PEG is removed from the final preparation as is shown by the absence of Peak 5 in all traces.
Figure 8.
Changes in the Molecular Weight of PEGylated Adenovirus Capsid Proteins Can be Detected by Gel Electrophoresis and Barium Iodide Staining. Unmodified (Lanes 4 and 5 both gels) and PEGylated (Lanes 2 and 3 both gels) adenovirus were boiled and run on 10% polyacrylamide gels with standard molecular weight markers (Lane 1 gel A) at 30 volts overnight. Duplicate samples were run such that, when electrophoresis was complete, the gel could be cut in half and either stained with Coomassie Brilliant Blue (Gel A) or a 5% barium chloride/1M iodine in 0.01 M perchloric acid (Gel B) for the identification of PEGylated proteins as described in reference . PEGylated proteins (Lanes 2 and 3, Gel A) are not stained as intensely as unmodified proteins (Lanes 4 and 5, Gel A) with Coomassie Blue despite the fact that the same amount of virus (based upon protein concentration) was loaded in each lane. In contrast, unmodified proteins were not resolved with barium chloride/iodide staining (Lanes 4 and 5, Gel B) while proteins with high PEG densities (Lanes 2 and 3, Gel B) could be detected by this method. Changes in the molecular weight of the adenovirus hexon (marked “a”), penton (marked “b”) and hexon-associated protein (marked “c”) were noted in the preparation included in the figure. The limit of detection of this assay was 0.5 μg of PEG in a 10% acrylamide gel.
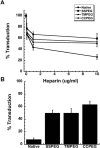
Figure 9.
(A) Effect of Heparin on Adenovirus Transduction Efficiency. Viruses were pre-incubated with heparin for 1 hour at 37 °C prior to addition to A549 cells. Virus binding was allowed to take place for 1 hour at 4 °C prior to the replacement of virus with culture medium. Values are representative of three separate experiments. Error bars represent the standard deviation of the data; (B) PEGylated Adenoviruses Enter Target Cells Partially Through Heparan Sulfate Glycosaminoglycans. Virus was pre-incubated with heparin (10 μg/ml) prior to addition to monolayers of A549 cells treated with both the anti-CAR antibody and a peptide which blocks integrin receptors (RmcB + RGD + Hep.). Percent transduction is the number of beta-galactosidase positive cells found in treated monolayers with respect to the number of positive cells found in monolayers that did not receive treatment prior to viral infection. Data are the average transduction efficiencies obtained from two separate experiments and error bars represent the standard deviation of the data.
Similar articles
- PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo.
O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE. O'Riordan CR, et al. Hum Gene Ther. 1999 May 20;10(8):1349-58. doi: 10.1089/10430349950018021. Hum Gene Ther. 1999. PMID: 10365665 - PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver.
Croyle MA, Chirmule N, Zhang Y, Wilson JM. Croyle MA, et al. Hum Gene Ther. 2002 Oct 10;13(15):1887-900. doi: 10.1089/104303402760372972. Hum Gene Ther. 2002. PMID: 12396620 - Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies.
Wortmann A, Vöhringer S, Engler T, Corjon S, Schirmbeck R, Reimann J, Kochanek S, Kreppel F. Wortmann A, et al. Mol Ther. 2008 Jan;16(1):154-62. doi: 10.1038/sj.mt.6300306. Epub 2007 Sep 11. Mol Ther. 2008. PMID: 17848961 - PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs.
Turecek PL, Bossard MJ, Schoetens F, Ivens IA. Turecek PL, et al. J Pharm Sci. 2016 Feb;105(2):460-475. doi: 10.1016/j.xphs.2015.11.015. J Pharm Sci. 2016. PMID: 26869412 Review. - Structure, biology, and therapeutic implications of pegylated interferon alpha-2b.
Youngster S, Wang YS, Grace M, Bausch J, Bordens R, Wyss DF. Youngster S, et al. Curr Pharm Des. 2002;8(24):2139-57. doi: 10.2174/1381612023393242. Curr Pharm Des. 2002. PMID: 12369859 Review.
Cited by
- Striving for Uniformity: A Review on Advances and Challenges To Achieve Uniform Polyethylene Glycol.
Bento C, Katz M, Santos MMM, Afonso CAM. Bento C, et al. Org Process Res Dev. 2024 Apr 1;28(4):860-890. doi: 10.1021/acs.oprd.3c00428. eCollection 2024 Apr 19. Org Process Res Dev. 2024. PMID: 38660381 Free PMC article. Review. - Challenges and progress toward tumor-targeted therapy by systemic delivery of polymer-complexed oncolytic adenoviruses.
Thambi T, Hong J, Yoon AR, Yun CO. Thambi T, et al. Cancer Gene Ther. 2022 Oct;29(10):1321-1331. doi: 10.1038/s41417-022-00469-y. Epub 2022 Apr 20. Cancer Gene Ther. 2022. PMID: 35444290 Free PMC article. Review. - Polymer stealthing and mucin-1 retargeting for enhanced pharmacokinetics of an oncolytic vaccinia virus.
Hill C, Grundy M, Bau L, Wallington S, Balkaran J, Ramos V, Fisher K, Seymour L, Coussios C, Carlisle R. Hill C, et al. Mol Ther Oncolytics. 2021 Mar 17;21:47-61. doi: 10.1016/j.omto.2021.03.011. eCollection 2021 Jun 25. Mol Ther Oncolytics. 2021. PMID: 33869742 Free PMC article. - Extracellular Vesicles-Mimetic Encapsulation Improves Oncolytic Viro-Immunotherapy in Tumors With Low Coxsackie and Adenovirus Receptor.
Zhang Y, Wu J, Zhang H, Wei J, Wu J. Zhang Y, et al. Front Bioeng Biotechnol. 2020 Sep 16;8:574007. doi: 10.3389/fbioe.2020.574007. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33042975 Free PMC article. - Protein cages and virus-like particles: from fundamental insight to biomimetic therapeutics.
Steinmetz NF, Lim S, Sainsbury F. Steinmetz NF, et al. Biomater Sci. 2020 May 19;8(10):2771-2777. doi: 10.1039/d0bm00159g. Biomater Sci. 2020. PMID: 32352101 Free PMC article. Review.
References
- Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54:459–476. - PubMed
- Smolinske SC. Handbook of Food, Drug, and Cosmetic Excipients. CRC Press; Boca Raton, FL, USA: 1992.
- Pasut G, Veronese FM. Polymer-drug conjugation, recent achievements and general strategies. Prog Polym Sci. 2007;32:933–961.
- Hamidi M, Azadi A, Rafiei P. Pharmacokinetic consequences of pegylation. Drug Deliv. 2006;13:399–409. - PubMed
- Ryan SM, Mantovani G, Wang X, Haddleton DM, Brayden DJ. Advances in PEGylation of important biotech molecules: delivery aspects. Expert Opin Drug Deliv. 2008;5:371–383. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources