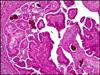
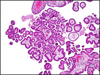
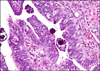
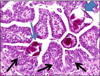
Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis - PubMed (original) (raw)
Multicenter Study
Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis
Robert J Kurman et al. Am J Surg Pathol. 2011 Nov.
Abstract
In contrast to the controversy regarding the terminology and behavior of ovarian noninvasive low-grade serous tumors [atypical proliferative serous tumor (APST) and serous borderline tumor], little attention has been directed to their origin. Similarly, until recently, proliferative lesions in the fallopian tube had not been extensively studied. The recent proposal that ovarian high-grade serous carcinomas are derived from intraepithelial carcinoma in the fallopian tube prompted us to evaluate the possible role of fallopian tube in the genesis of low-grade serous tumors. We have identified a lesion, designated "papillary tubal hyperplasia (PTH)," characterized by small rounded clusters of tubal epithelial cells and small papillae, with or without associated psammoma bodies, that are present within the tubal lumen and which are frequently associated with APSTs. Twenty-two cases in this study were selected from a population-based study in Denmark of approximately 1000 patients with low-grade ovarian serous tumors in whom implants were identified on the fallopian tube. Seven additional cases were seen recently in consultation at The Johns Hopkins Hospital (JHH). These 7 cases were not associated with an ovarian tumor. PTH was found in 20 (91%) of the 22 cases in the Danish study. On the basis of this association of PTH with APSTs with implants and the close morphologic resemblance of PTH, not only to primary ovarian APSTs but also to noninvasive epithelial implants and endosalpingiosis, we speculate that the small papillae and clusters of cells from the fallopian tube implant on ovarian and peritoneal surfaces to produce these lesions. The 7 JHH cases of PTH that were not associated with an ovarian tumor support the view that PTH is the likely precursor lesion. We propose a model for the development of ovarian and extraovarian low-grade serous proliferations (APST, noninvasive epithelial implants, and endosalpingiosis) that postulates that all of these lesions are derived from PTH, which appears to be induced by chronic inflammation. If this hypothesis is confirmed, it can be concluded that low-grade and high-grade ovarian tumors develop from tubal epithelium and involve the ovary secondarily.
Figures
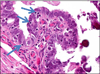
Fig. 1
A Papillary tubal hyperplasia. Although the overall plical architectural changes are subtle, the plicae are slightly thicker and the overall appearance looks “busy”. B Normal fallopian tube.
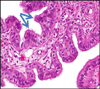
Fig. 2
Papillary tubal hyperplasia. Numerous small papillae and psammoma bodies in the tubal lumen.
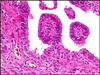
Fig. 3
Papillary tubal hyperplasia. Multiple small papillae floating in tubal lumen.
Fig. 4
Papillary tubal hyperplasia. A profuse number of small papillae in the tubal lumen.
Fig. 5
Papillary tubal hyperplasia. A “naked” psammoma body lying on surface of tubal epithelium and another one within the core of a small papilla, so-called salpingolith.
Fig. 6
Papillary tubal hyperplasia. Psammoma bodies within a papilla (salpingolith), tubal epithelium (blue thin arrow) and lamina propria (blue block arrow). Numerous intraepithelial lymphocytes are located just above the basement membrane in the mucosa (black arrows).
Fig. 7
Papillary tubal hyperplasia. Papilla arising from mucosa before it becomes pinched off. Secretory cells lie between ciliated cells. Intraepithelial lymphocytes lie just above the basement membrane (arrows). The nucleus is characteristically surrounded by a halo.
Fig. 8
Early tubal hyperplasia. Small elevated tufts of epithelium (arrows) represent the earliest hyperplastic change.
Fig. 9
Early tubal hyperplasia. Papillary bud still attached to tubal mucosa appears to be the next stage of hyperplasia before being pinched off and floating into the tubal lumen.
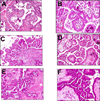
Fig. 10
A and B. Papillary tubal hyperplasia; C and D. Noninvasive epithelial implant; E and F. Atypical proliferative (borderline) serous tumor. Images are from different cases.
Similar articles
- Mucosal Proliferations in Completely Examined Fallopian Tubes Accompanying Ovarian Low-grade Serous Tumors: Neoplastic Precursor Lesions or Normal Variants of Benign Mucosa?
Wolsky RJ, Price MA, Zaloudek CJ, Rabban JT. Wolsky RJ, et al. Int J Gynecol Pathol. 2018 May;37(3):262-274. doi: 10.1097/PGP.0000000000000410. Int J Gynecol Pathol. 2018. PMID: 28700429 - [Morphologic changes of fallopian tubal epithelium in ovarian serous tumors].
Wen J, Shi JL, Shen DH, Chen YX, Song QJ. Wen J, et al. Zhonghua Bing Li Xue Za Zhi. 2012 Jul;41(7):433-7. doi: 10.3760/cma.j.issn.0529-5807.2012.07.001. Zhonghua Bing Li Xue Za Zhi. 2012. PMID: 22932451 Chinese. - Fallopian tube precursors of ovarian low- and high-grade serous neoplasms.
Vang R, Shih IeM, Kurman RJ. Vang R, et al. Histopathology. 2013 Jan;62(1):44-58. doi: 10.1111/his.12046. Histopathology. 2013. PMID: 23240669 Review. - Evidence for lineage continuity between early serous proliferations (ESPs) in the Fallopian tube and disseminated high-grade serous carcinomas.
Soong TR, Howitt BE, Miron A, Horowitz NS, Campbell F, Feltmate CM, Muto MG, Berkowitz RS, Nucci MR, Xian W, Crum CP. Soong TR, et al. J Pathol. 2018 Nov;246(3):344-351. doi: 10.1002/path.5145. Epub 2018 Sep 27. J Pathol. 2018. PMID: 30043522 - Taking the Tube: From Normal Fallopian Tube Epithelium to Ovarian High-grade Serous Carcinoma.
Tone AA. Tone AA. Clin Obstet Gynecol. 2017 Dec;60(4):697-710. doi: 10.1097/GRF.0000000000000313. Clin Obstet Gynecol. 2017. PMID: 29045296 Review.
Cited by
- The role of the fallopian tube in the origin of ovarian cancer.
Erickson BK, Conner MG, Landen CN Jr. Erickson BK, et al. Am J Obstet Gynecol. 2013 Nov;209(5):409-14. doi: 10.1016/j.ajog.2013.04.019. Epub 2013 Apr 10. Am J Obstet Gynecol. 2013. PMID: 23583217 Free PMC article. Review. - Early preinvasive lesions in ovarian cancer.
Chene G, Lamblin G, Le Bail-Carval K, Chabert P, Bakrin N, Mellier G. Chene G, et al. Biomed Res Int. 2014;2014:639252. doi: 10.1155/2014/639252. Epub 2014 Apr 8. Biomed Res Int. 2014. PMID: 24804229 Free PMC article. Review. - Concurrent isolated retroperitoneal HGSC and STIC defined by somatic mutation analysis: a case report.
Suda K, Nakaoka H, Hata C, Yahata N, Isobe M, Kameyama H, Wakai T, Motoyama T, Inoue I, Yoshihara K, Enomoto T. Suda K, et al. Diagn Pathol. 2019 Feb 11;14(1):17. doi: 10.1186/s13000-019-0795-3. Diagn Pathol. 2019. PMID: 30744657 Free PMC article. - Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants.
Ardighieri L, Zeppernick F, Hannibal CG, Vang R, Cope L, Junge J, Kjaer SK, Kurman RJ, Shih IeM. Ardighieri L, et al. J Pathol. 2014 Jan;232(1):16-22. doi: 10.1002/path.4293. J Pathol. 2014. PMID: 24307542 Free PMC article. - Subtypes of Ovarian Cancer and Ovarian Cancer Screening.
Koshiyama M, Matsumura N, Konishi I. Koshiyama M, et al. Diagnostics (Basel). 2017 Mar 2;7(1):12. doi: 10.3390/diagnostics7010012. Diagnostics (Basel). 2017. PMID: 28257098 Free PMC article. Review.
References
- Beckman EN, Pintado SU, Leonard GL, Sternberg WH. Endometriosis of the prostate. Am J Surg Pathol. 1985;9:374–379. - PubMed
- Bell DA, Scully RE. Serous borderline tumors of the peritoneum. Am J Surg Pathol. 1990;14:230–239. - PubMed
- Dougherty CM, Cotten NM. Proliferative epithelial lesions of the uterine tube. Am J Obstet Gynecol. 1964;24:849–854. - PubMed
- Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical