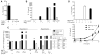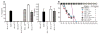Successful transmission of a retrovirus depends on the commensal microbiota - PubMed (original) (raw)
Successful transmission of a retrovirus depends on the commensal microbiota
Melissa Kane et al. Science. 2011.
Abstract
To establish chronic infections, viruses must develop strategies to evade the host's immune responses. Many retroviruses, including mouse mammary tumor virus (MMTV), are transmitted most efficiently through mucosal surfaces rich in microbiota. We found that MMTV, when ingested by newborn mice, stimulates a state of unresponsiveness toward viral antigens. This process required the intestinal microbiota, as antibiotic-treated mice or germ-free mice did not transmit infectious virus to their offspring. MMTV-bound bacterial lipopolysaccharide triggered Toll-like receptor 4 and subsequent interleukin-6 (IL-6)-dependent induction of the inhibitory cytokine IL-10. Thus, MMTV has evolved to rely on the interaction with the microbiota to induce an immune evasion pathway. Together, these findings reveal the fundamental importance of commensal microbiota in viral infections.
Figures
Fig. 1
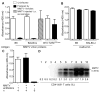
Unresponsiveness to MMTV is dependent on the oral route of infection and on microbiota. (A and B) Mice of indicated strains—MMTV-free or infected by fostering on MMTV-infected lactating MMTV(LA) females or infected intraperitoneally (i.p.) at 3 to 5 days of age—were immunized at 8 weeks of age with either MMTV(LA) proteins (A) or ovalbumin (B) in Freund’s complete adjuvant. Production of specific Abs to respective antigens was tested by enzyme-linked immunosorbent assay (ELISA). Sera from four to eight mice were used per group. One of three experiments is shown. (C) The progeny from the first pregnancy of uninfected and MMTV(LA)-infected B6 females, treated with a mixture of broad-spectrum antibiotics or left untreated, were immunized at 6 weeks of age with MMTV(LA) proteins and tested for virus-specific Abs by ELISA (detailed experimental scheme is shown in fig. S1A). n, number of mice. All graphs, means ± SEM. (D) MMTV infection of mice used in (C) was probed by polymerase chain reaction (PCR) amplification (under saturating conditions) of integrated proviruses in splenic DNA followed by Southern blot with an MMTV long terminal repeat (LTR)–specific probe (lanes 1 to 4, offspring of infected antibiotic-treated females; lanes 5 to 10, offspring of infected, untreated females) and by evaluating deletion of SAg-cognate CD4+ Vμ6+ T cells. Uninfected B6 mice (n = 5) had 8.5 ± 0.3% of CD4+Vμ6+ T cells among CD4+ T cells.
Fig. 2
Differential MMTV persistence in GF and SPF mice of distinct genotypes. (A) Reverse transcription–PCR detection of MMTV virion RNA in the milk of GF C3H/HeN mice infected parenterally with MMTV(C3H). NC, negative control: RNA from the milk of an uninfected C3H/HeN mouse; PC, positive control: RNA from the milk of an SPF mouse injected with the same viral isolate. MMTV(C3H) and MMTV(LA) were used in two independent studies, data with MMTV(C3H) are shown. To ensure sufficient time for virus amplification in injected mice, the second litters by these dams were used for breeding and testing. (B) Splenic DNA from the first progeny (G1) of i.p. injected mice (G0) was subjected to MMTV-specific PCR, followed by Southern blot hybridization with an MMTV LTR–specific probe. Splenic DNA from an SPF G1 progeny of mice injected with the same virus isolate was used as PC, splenic DNA from an MMTV-negative C3H/HeN mouse was used as NC. a and b, independent families. The figure is assembled from nonconcurrent portions of the same image. (C) Virus fate in the offspring of parenterally infected (G0) mice of different genotypes and maintenance conditions. Summary of data from MMTV-infected mice, showing virus loss or persistence at G1. (D) Transmission of infectious MMTV(C3H) in GF MyD88KOTLR4Lps-del mice. Detection of integrated proviruses was done as in (B). NC, DNA from the spleen of an uninfected C3H/HeN mouse. A single representative family (a) is shown. (E) Transmission of infectious MMTV(C3H) in ASF-associated gnotobiotic wild-type C3H/HeN mice as detected by MMTV(C3H)-specific PCR, followed by Southern blot analysis, performed with splenic DNA of mice from G0 to G2. a to c, different families. Splenic DNA from SPF G1 progeny of mice injected with the same virus isolate served as PC, and splenic DNA from an MMTV-negative C3H/HeN mouse was used as NC. For (A), (B), (D), and (E), PCRs were performed under saturating conditions.
Fig. 3
MMTV-bound LPS induces production of IL-10. (A) Splenocytes from wild-type C3H/HeN (wt) or C3H/HeN TLR4Lps-del (Lps-del) mice were incubated with an MMTV-SPF isolate in the presence or absence of 0.1 μg/ml PMB. IL-10 was detected in tissue-culture supernatants by ELISA 16 hours later. Graph shows means ± SEM from four independent experiments. (B) Splenocytes from wild-type C3H/HeN mice were incubated with different concentrations of LPS with or without 0.1 μg/ml PMB followed by IL-10 detection as in (A). In parallel, cells were exposed to an MMTV-SPF isolate in the presence or absence of 0.1 μg/ml PMB. Concentration of LPS in the cultures containing the MMTV-SPF isolate was ~13 ng/ml as determined by LAL assay. Graph shows means ± SEM. One of three experiments shown. (C) MMTV-SPF or MMTV-GF virions were ultracentrifuged through 30% sucrose with a phosphate-buffered saline (PBS) cushion; the pellet, PBS supernatant (PBS fraction), and initial “before spin” fraction were assayed for LPS by LAL assay. In parallel, indicated amounts of LPS (from Escherichia coli serotype 026:B6) incubated with PBS or MMTV-GF were ultracentrifuged through a sucrose cushion and similarly tested. Several fractions (samples a to f) were tested for the ability to elicit IL-10 in in vitro cultures of C3H/HeN splenocytes (IL-10 and final LPS concentrations in the in vitro cultures are shown in the table). LPS added: total amount of LPS added to MMTV-GF or PBS before centrifugation. ND, not determined. Graph shows means ± SEM. (D) Four independent MMTV-GF isolates (either LPS-free or containing LPS at 1 pg/ml) alone with unbound LPS were compared for their ability to induce IL-10 secretion by splenocytes (left). All virus isolates were normalized by ELISA (not shown). MMTV-SPF at several dilutions, MMTV-GF mixed with various concentrations of LPS, and the same concentrations of free LPS were compared for their ability to induce IL-10 in splenocyte cultures. One of three experiments is shown (right). Graphs show means ± SEM.
Fig. 4
Genetic delineation of the immune subversion pathway induced by MMTV-LPS triggering of TLR4. (A) Splenocytes from B6 or B10 wild-type (used interchangeably), or from indicated knockout or mutant mice were incubated with an MMTV-SPF isolate followed by detection of IL-6 and IL-10 in supernatants by ELISA. Three to five mice were used per group. Graphs show means ± SEM. (B) Virus elimination in subsequent generations of mice with deficiencies within the immune sub-version pathway. G0 mice were fostered by SPF MMTV(LA)-infected C3H/HeN females. At least three animals per family were analyzed for hallmarks of infection: deletion of SAg-cognate T cells, viral RNA in the milk (table S1 and fig. S8A), and integrated proviruses in spleens (fig. S8B). A family that eliminated the virus was allowed to produce the next generation of mice, which was also tested to confirm virus loss. MMTV(LA)-infected B6 and B10 mice were used as controls. To control for background modifiers, 129/SvJ mice were also included, as many targeted knockout mice were originally generated on the 129/SvJ background.
Comment in
- Microbiology. Gut bacteria lend a molecular hand to viruses.
Pennisi E. Pennisi E. Science. 2011 Oct 14;334(6053):168. doi: 10.1126/science.334.6053.168. Science. 2011. PMID: 21998362 No abstract available. - Viral infection. The gut microbiota: friend or foe?
David R. David R. Nat Rev Microbiol. 2011 Nov 2;9(12):831. doi: 10.1038/nrmicro2691. Nat Rev Microbiol. 2011. PMID: 22048739
Similar articles
- Microbiology. Gut bacteria lend a molecular hand to viruses.
Pennisi E. Pennisi E. Science. 2011 Oct 14;334(6053):168. doi: 10.1126/science.334.6053.168. Science. 2011. PMID: 21998362 No abstract available. - B and T cells are required for mouse mammary tumor virus spread within the mammary gland.
Golovkina TV, Dudley JP, Ross SR. Golovkina TV, et al. J Immunol. 1998 Sep 1;161(5):2375-82. J Immunol. 1998. PMID: 9725233 - Subversion of the innate immune system by a retrovirus.
Jude BA, Pobezinskaya Y, Bishop J, Parke S, Medzhitov RM, Chervonsky AV, Golovkina TV. Jude BA, et al. Nat Immunol. 2003 Jun;4(6):573-8. doi: 10.1038/ni926. Epub 2003 May 5. Nat Immunol. 2003. PMID: 12730691 - Mouse mammary tumor virus and the immune system.
Czarneski J, Rassa JC, Ross SR. Czarneski J, et al. Immunol Res. 2003;27(2-3):469-80. doi: 10.1385/IR:27:2-3:469. Immunol Res. 2003. PMID: 12857990 Review. - Immune response to MMTV infection.
Acha-Orbea H, Shakhov AN, Finke D. Acha-Orbea H, et al. Front Biosci. 2007 Jan 1;12:1594-609. doi: 10.2741/2172. Front Biosci. 2007. PMID: 17127406 Review.
Cited by
- The microbiome and innate immunity.
Thaiss CA, Zmora N, Levy M, Elinav E. Thaiss CA, et al. Nature. 2016 Jul 7;535(7610):65-74. doi: 10.1038/nature18847. Nature. 2016. PMID: 27383981 Review. - Microbiota and Its Role on Viral Evasion: Is It With Us or Against Us?
Domínguez-Díaz C, García-Orozco A, Riera-Leal A, Padilla-Arellano JR, Fafutis-Morris M. Domínguez-Díaz C, et al. Front Cell Infect Microbiol. 2019 Jul 18;9:256. doi: 10.3389/fcimb.2019.00256. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31380299 Free PMC article. Review. - Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus.
Robinson CM, Jesudhasan PR, Pfeiffer JK. Robinson CM, et al. Cell Host Microbe. 2014 Jan 15;15(1):36-46. doi: 10.1016/j.chom.2013.12.004. Cell Host Microbe. 2014. PMID: 24439896 Free PMC article. - An Update on the Mutual Impact between SARS-CoV-2 Infection and Gut Microbiota.
Li S, Zhou Y, Yan D, Wan Y. Li S, et al. Viruses. 2022 Aug 15;14(8):1774. doi: 10.3390/v14081774. Viruses. 2022. PMID: 36016396 Free PMC article. Review. - Autophagy, viruses, and intestinal immunity.
Kernbauer E, Cadwell K. Kernbauer E, et al. Curr Opin Gastroenterol. 2014 Nov;30(6):539-46. doi: 10.1097/MOG.0000000000000121. Curr Opin Gastroenterol. 2014. PMID: 25291356 Free PMC article. Review.
References
- Malim MH, Emerman M. Cell Host Microbe. 2008;3:388. - PubMed
- Dittmer U, et al. Immunity. 2004;20:293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI090084/AI/NIAID NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- R01 AI090084/AI/NIAID NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- DK42086/DK/NIDDK NIH HHS/United States
- T32 AI065382-01/AI/NIAID NIH HHS/United States
- R01 AI082418/AI/NIAID NIH HHS/United States
- AI082418/AI/NIAID NIH HHS/United States
- R01 CA134667/CA/NCI NIH HHS/United States
- R56 AI090084/AI/NIAID NIH HHS/United States
- T32GM007183/GM/NIGMS NIH HHS/United States
- CA100383/CA/NCI NIH HHS/United States
- R01 CA100383/CA/NCI NIH HHS/United States
- T32 GM007183/GM/NIGMS NIH HHS/United States
- T32 AI065382/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources