Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness - PubMed (original) (raw)
Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness
Gary D Slade et al. Pain. 2011 Dec.
Abstract
For reasons unknown, temporomandibular disorder (TMD) can manifest as localized pain or in conjunction with widespread pain. We evaluated relationships between cytokines and TMD without or with widespread palpation tenderness (TMD-WPT or TMD+WPT, respectively) at protein, transcription factory activity, and gene levels. Additionally, we evaluated the relationship between cytokines and intermediate phenotypes characteristic of TMD and WPT. In a case-control study of 344 females, blood samples were analyzed for levels of 22 cytokines and activity of 48 transcription factors. Intermediate phenotypes were measured by quantitative sensory testing and questionnaires asking about pain, health, and psychological status. Single nucleotide polymorphisms (SNPs) coding cytokines and transcription factors were genotyped. TMD-WPT cases had elevated protein levels of proinflammatory cytokine monocyte chemotactic protein (MCP-1) and antiinflammatory cytokine interleukin (IL)-1ra, whereas TMD+WPT cases had elevated levels of proinflammatory cytokine IL-8. MCP-1, IL-1ra, and IL-8 were differentially associated with experimental pain, self-rated pain, self-rated health, and psychological phenotypes. TMD-WPT and TMD+WPT cases had inhibited transcription activity of the antiinflammatory cytokine transforming growth factor β1 (TGFβ1). Interactions were observed between TGFβ1 and IL-8 SNPs: an additional copy of the TGFβ1 rs2241719 minor T allele was associated with twice the odds of TMD+WPT among individuals homozygous for the IL-8 rs4073 major A allele, and half the odds of TMD+WPT among individuals heterozygous for rs4073. These results demonstrate how pro- and antiinflammatory cytokines contribute to the pathophysiology of TMD and WPT in genetically susceptible people. Furthermore, they identify MCP-1, IL-1ra, IL-8, and TGFβ1 as potential diagnostic markers and therapeutic targets for pain in patients with TMD.
Published by Elsevier B.V.
Conflict of interest statement
Statement of conflict of interest. This study includes results from genotyping that was performed using the Algynomics Pain Research Panel. Algynomics Inc. is a company providing research services in personalized pain medication and diagnostics. Five authors have interests in Algynomics Inc. which may be relevant in determining conflicts of interest. Specifically, Drs. Slade, Diatchenko, Smith, Maixner and Nackly hold shares and/or stock options in Algynomics Inc. Also, Drs. Diatchenko and Maixner are Office-holders in Algyonomics Inc. All other authors report no known conflicts of interest.
Figures
Fig. 1
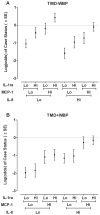
Circulating cytokine protein signatures differ among TMD−WPT and TMD+WPT cases. MCP-1 and IL-1ra are TMD-specific as their levels are elevated in TMD−WPT, but not in TMD+WPT cases. IL-8 is TMD+WPT-specific as its levels are elevated in TMD+WPT, but not in TMD−WPT cases. N = 344. Data are Mean ± SEM. *P<0.002, the critical P-value after Bonferroni correction testing for differences between each case group versus the control group.
Fig. 2
Circulating cytokine levels are differentially associated with log-odds of TMD−WPT and TMD+WPT. (A) Both MCP-1 and IL-1ra are independently associated with log-odds of TMD−WPT, whereas IL-8 is not. (B) In contrast, IL-8 and MCP-1 are associated with odds of TMD+WPT, while IL-1ra is not. Predicted odds, plotted on the log scale, was calculated for each case classification relative to controls using parameter estimates from a multivariable generalized logit model shown in Table 3 (N=344 females). Cytokine protein levels were modelled as continuous variables, each transformed to unit normal deviates. “Lo” signifies a value of one standard deviation below the mean for the cytokine and “Hi” signifies a value of one standard deviation above the mean for the cytokine.
Fig. 3
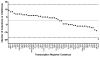
TGFβ1 transcription activity is altered among TMD−WPT and TMD+WPT cases. Fold-change in transcription factor activities for TMD−WPT and TMD+WPT cases across cell type and experimental condition are shown. TGFβ1 activity was consistently inhibited in cases relative to controls in 24 activity profiles. Fold-inductions or fold-inhibitions among the remaining 47 transcription reporter constructs did not differ between cases and controls. Data are the number of fold-inductions (values above 0) or fold-inhibitions (values below 0) out of 32 activity profiles per transcription factor in TMD−WPT and TMD+WPT cases relative to healthy controls. The solid line set at 0 represents no change relative to controls, while the dashed lines set at 22 and −22 represent cut-offs corresponding to the number of inductions or inhibitions expected by chance based on a two-tailed binomial probability test. The value for TGFβ1 fell outside the boundaries of the chance expectation, and therefore is statistically significant (P<0.05).
Fig. 4
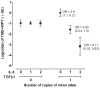
IL-8 and TGFβ1 SNPs interact to affect log-odds of TMD+WPT. Predicted log-odds of TMD+WPT is from a logistic regression model testing for effects of IL-8 and TGFβ1 SNPs, each modeled as the number of copies of the minor alllele and the interaction between the two SNPs (N=344 females). Contrasting effects of IL-8 on case status were apparent. Log-odds of TMD+WPT was not associated with the number of copies of the minor A allele of IL-8 rs4073 among heterozygotes of the major A allele of TGFβ1 rs2241719. In contrast, log-odds of TMD+WPT was markedly reduced with each additional copy of the minor A allele of IL-8 rs4073 among heterozygotes of the major A allele of TGFβ1 rs2241719. Likewise, contrasting effects of TGFβ1 on case status were apparent. Among females with 0 copies of the minor A allele of IL-8 rs4073 (●), an additional copy of the minor T allele of TGFβ1 rs2241719 was associated with an increase of 0.97 in the log-odds of TMD+WPT. This is equivalent to an odds ratio of 2.6 (95%CI = 1.6, 6.2) for the association of the T allele of TGFβ1 with TMD+WPT among homozygotes for the major A allele of IL-8 rs4073. In contrast, among females with 1 copy of the minor A allele of IL-8 rs4073 (◆), an additional copy of the minor T allele of TGFβ1 rs2241719 was associated with a decrease of 0.60 in the log-odds of TMD+WPT. This is equivalent to an odds ratio of 0.55 (95%CI = 0.24, 1.3) for the association of the T allele of TGFβ1 with TMD+WPT among heterozygotes for IL-8 rs4073. Among females with 2 copies of the minor A allele of IL-8 rs4073 (■), there was a corresponding reduction of 2.2 in log-odds of TMD+WPT, equivalent to an odds ratio of 0.11 (95% CI = 0.02, 1.3). Only eight individuals were homozygous for the minor allele T of TGFβ1 rs2241719, so data for that genotype are not plotted.
Similar articles
- Relationship between temporomandibular disorders, widespread palpation tenderness, and multiple pain conditions: a case-control study.
Chen H, Slade G, Lim PF, Miller V, Maixner W, Diatchenko L. Chen H, et al. J Pain. 2012 Oct;13(10):1016-27. doi: 10.1016/j.jpain.2012.07.011. J Pain. 2012. PMID: 23031401 Free PMC article. - Inflammatory Cytokines and Sleep Disturbance in Patients with Temporomandibular Disorders.
Park JW, Chung JW. Park JW, et al. J Oral Facial Pain Headache. 2016 Winter;30(1):27-33. doi: 10.11607/ofph.1367. J Oral Facial Pain Headache. 2016. PMID: 26817030 - The IL-1Ra gene variable number tandem repeat variant is associated with susceptibility to temporomandibular disorders in Turkish population.
Tumer MK, Nursal AF, Tekcan A, Yerliyurt K, Geyko A, Yigit S. Tumer MK, et al. J Clin Lab Anal. 2018 Feb;32(2):e22255. doi: 10.1002/jcla.22255. Epub 2017 Jun 14. J Clin Lab Anal. 2018. PMID: 28612927 Free PMC article. - Inflammation and bony changes at the temporomandibular joint.
Kacena MA, Merrel GA, Konda SR, Wilson KM, Xi Y, Horowitz MC. Kacena MA, et al. Cells Tissues Organs. 2001;169(3):257-64. doi: 10.1159/000047889. Cells Tissues Organs. 2001. PMID: 11455121 Review. - Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies.
Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, Dubner R, Diatchenko L, Meloto CB, Smith S, Maixner W. Slade GD, et al. J Dent Res. 2016 Sep;95(10):1084-92. doi: 10.1177/0022034516653743. Epub 2016 Jun 23. J Dent Res. 2016. PMID: 27339423 Free PMC article. Review.
Cited by
- Temporomandibular disorder, body pain and systemic diseases: assessing their associations in adolescents.
Braido GVDV, Campi LB, Jordani PC, Fernandes G, GonÇalves DAG. Braido GVDV, et al. J Appl Oral Sci. 2020 Sep 7;28:e20190608. doi: 10.1590/1678-7757-2019-0608. J Appl Oral Sci. 2020. PMID: 32901693 Free PMC article. - Xanthan gum protects temporomandibular chondrocytes from IL‑1β through Pin1/NF‑κB signaling pathway.
Yuan F, Xie JL, Liu KY, Shan JL, Sun YG, Ying WG. Yuan F, et al. Mol Med Rep. 2020 Aug;22(2):1129-1136. doi: 10.3892/mmr.2020.11233. Epub 2020 Jun 15. Mol Med Rep. 2020. PMID: 32626995 Free PMC article. - Genome-wide association reveals contribution of MRAS to painful temporomandibular disorder in males.
Smith SB, Parisien M, Bair E, Belfer I, Chabot-Doré AJ, Gris P, Khoury S, Tansley S, Torosyan Y, Zaykin DV, Bernhardt O, de Oliveira Serrano P, Gracely RH, Jain D, Järvelin MR, Kaste LM, Kerr KF, Kocher T, Lähdesmäki R, Laniado N, Laurie CC, Laurie CA, Männikkö M, Meloto CB, Nackley AG, Nelson SC, Pesonen P, Ribeiro-Dasilva MC, Rizzatti-Barbosa CM, Sanders AE, Schwahn C, Sipilä K, Sofer T, Teumer A, Mogil JS, Fillingim RB, Greenspan JD, Ohrbach R, Slade GD, Maixner W, Diatchenko L. Smith SB, et al. Pain. 2019 Mar;160(3):579-591. doi: 10.1097/j.pain.0000000000001438. Pain. 2019. PMID: 30431558 Free PMC article. - Biomarkers for Temporomandibular Disorders: Current Status and Future Directions.
Zwiri A, Al-Hatamleh MAI, W Ahmad WMA, Ahmed Asif J, Khoo SP, Husein A, Ab-Ghani Z, Kassim NK. Zwiri A, et al. Diagnostics (Basel). 2020 May 15;10(5):303. doi: 10.3390/diagnostics10050303. Diagnostics (Basel). 2020. PMID: 32429070 Free PMC article. Review. - Differential expression of systemic inflammatory mediators in amputees with chronic residual limb pain.
Chamessian A, Van de Ven T, Buchheit T, Hsia HL, McDuffie M, Gamazon ER, Walsh C, Bruehl S, Buckenmaier C' 3rd, Shaw A. Chamessian A, et al. Pain. 2017 Jan;158(1):68-74. doi: 10.1097/j.pain.0000000000000728. Pain. 2017. PMID: 27682210 Free PMC article.
References
- Abraham S, Sawaya BE, Safak M, Batuman O, Khalili K, Amini S. Regulation of MCP-1 gene transcription by Smads and HIV-1 Tat in human glial cells. Virology. 2003;309(2):196–202. - PubMed
- Ahn DK, Lee KR, Lee HJ, Kim SK, Choi HS, Lim EJ, Park JS. Intracisternal administration of chemokines facilitated formalin-induced behavioral responses in the orofacial area of freely moving rats. Brain Res Bull. 2005;66(1):50–58. - PubMed
- Allerbring M, Haegerstam G. Characteristics of patients with chronic idiopathic orofacial pain. A retrospective study. Acta Odontol Scand. 1993;51(1):53–58. - PubMed
- Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS072205/NS/NINDS NIH HHS/United States
- R01 DE016558/DE/NIDCR NIH HHS/United States
- K12 HD052191/HD/NICHD NIH HHS/United States
- R01 NS072205-01A1/NS/NINDS NIH HHS/United States
- P01 NS045685/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous