Posttranslational regulation of AMPA receptor trafficking and function - PubMed (original) (raw)
Review
Posttranslational regulation of AMPA receptor trafficking and function
Wei Lu et al. Curr Opin Neurobiol. 2012 Jun.
Abstract
In the mammalian central nervous system, the majority of fast excitatory synaptic transmission is mediated by glutamate acting on AMPA-type ionotropic glutamate receptors. The abundance of AMPA receptors at the synapse can be modulated through receptor trafficking, which dynamically regulates many fundamental brain functions, including learning and memory. Reversible posttranslational modifications, including phosphorylation, palmitoylation and ubiquitination of AMPA receptor subunits are important regulatory mechanisms for controlling synaptic AMPA receptor expression and function. In this review, we highlight recent advances in the study of AMPA receptor posttranslational modifications and discuss how these modifications regulate AMPA receptor trafficking and function at synapses.
Published by Elsevier Ltd.
Figures
Figure 1
Topology and posttranslational modification sites of AMPA receptor subunits Each AMPA receptor subunit is an integral membrane protein with an extracellular amino terminal domain (NTD), three transmembrane domains (M1, 3 and 4), a hydrophobic hairpin domain (M2), which underlies the formation of the channel pore, and three intracellular domains, Loop1, Loop2 and the carboxyl-tail (C-tail). The S1 domain in the N-terminus and the S2 domain that links M3 and M4 underlie the formation of glutamate binding domain. (a) Schematic drawings of the GluA1 subunit. The residues that are palmitoylated (in green), phosphorylated (in red) and ubiquitinated (in purple) are highlighted. (b) Schematic drawings of the GluA2 subunit. The residues that are palmitoylated (in green) and phosphorylated (in red) are highlighted. Sub-domains that mediate the interaction with 4.1N protein (a) or GRIP/ABP and PICK1 (b) are also indicated (in yellow).
Figure 2
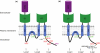
Phosphorylation of GluA1 regulates AMPA receptor trafficking. (a) Phosphorylation on S818, S831 and S845 of the GluA1 C-tail enhances delivery of the receptor to the neuronal surface. Among these phosphorylation events, phosphorylation at S818 facilitates the GluA1 interaction with 4.1N protein, which stabilizes the receptor on the surface. Conversely, dephosphorylation, especially at S845, appears to be important for receptor endocytosis. (b) Phosphorylation on S567 in the GluA1 Loop1 domain negatively regulates AMPA receptor trafficking to synapses and thus dephosphorylation at this site is a prerequisite for synaptic insertion of the receptor. The arrows (brown) represent specific steps of AMPA receptor trafficking.
Figure 3
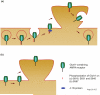
Palmitoylation of the AMPA receptor plays versatile roles in receptor trafficking. Palmitoylation at C585 in the GluA1 M2 hairpin structure takes place at the endoplasmic reticulum (ER) and functions in stabilizing the receptor in the ER. Once the receptor is trafficked to the Golgi apparatus, depalmitoylation at C585 is required for forward trafficking of the receptor. Palmitoylation of GluA1 at C811 in the C-tail inhibits nearby PKC phosphorylation on S818. Phosphorylation of S818 enhances the GluA1 interaction with 4.1N protein, thus stabilizing the receptor on the surface. Therefore, palmitoylation of C811 negatively regulates the GluA1-4.1N interaction, facilitating stimulation-induced endocytosis of the AMPA receptor. The arrows (brown) represent specific steps of AMPA receptor trafficking.
Similar articles
- Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity.
Jiang J, Suppiramaniam V, Wooten MW. Jiang J, et al. Neurosignals. 2006-2007;15(5):266-82. doi: 10.1159/000105517. Epub 2007 Jul 9. Neurosignals. 2006. PMID: 17622793 Review. - Auxiliary subunits provide new insights into regulation of AMPA receptor trafficking.
Sumioka A. Sumioka A. J Biochem. 2013 Apr;153(4):331-7. doi: 10.1093/jb/mvt015. Epub 2013 Feb 20. J Biochem. 2013. PMID: 23426437 Review. - S-palmitoylation regulates AMPA receptors trafficking and function: a novel insight into synaptic regulation and therapeutics.
Han J, Wu P, Wang F, Chen J. Han J, et al. Acta Pharm Sin B. 2015 Jan;5(1):1-7. doi: 10.1016/j.apsb.2014.12.002. Epub 2014 Dec 29. Acta Pharm Sin B. 2015. PMID: 26579419 Free PMC article. Review. - AMPAR trafficking in synapse maturation and plasticity.
Bassani S, Folci A, Zapata J, Passafaro M. Bassani S, et al. Cell Mol Life Sci. 2013 Dec;70(23):4411-30. doi: 10.1007/s00018-013-1309-1. Epub 2013 Mar 9. Cell Mol Life Sci. 2013. PMID: 23475111 Free PMC article. Review. - AMPA receptor trafficking at excitatory synapses.
Bredt DS, Nicoll RA. Bredt DS, et al. Neuron. 2003 Oct 9;40(2):361-79. doi: 10.1016/s0896-6273(03)00640-8. Neuron. 2003. PMID: 14556714 Review.
Cited by
- Flip-flopping to the membrane.
Salussolia CL, Wollmuth LP. Salussolia CL, et al. Neuron. 2012 Nov 8;76(3):463-5. doi: 10.1016/j.neuron.2012.10.022. Neuron. 2012. PMID: 23141057 Free PMC article. - In the fast lane: Receptor trafficking during status epilepticus.
Naylor DE. Naylor DE. Epilepsia Open. 2023 May;8 Suppl 1(Suppl 1):S35-S65. doi: 10.1002/epi4.12718. Epub 2023 Mar 20. Epilepsia Open. 2023. PMID: 36861477 Free PMC article. Review. - GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex.
Qiu S, Zhang M, Liu Y, Guo Y, Zhao H, Song Q, Zhao M, Huganir RL, Luo J, Xu H, Zhuo M. Qiu S, et al. J Neurosci. 2014 Oct 1;34(40):13505-15. doi: 10.1523/JNEUROSCI.1431-14.2014. J Neurosci. 2014. PMID: 25274827 Free PMC article. - Plasticity-induced actin polymerization in the dendritic shaft regulates intracellular AMPA receptor trafficking.
Wong VC, Houlihan PR, Liu H, Walpita D, DeSantis MC, Liu Z, O'Shea EK. Wong VC, et al. Elife. 2024 Aug 15;13:e80622. doi: 10.7554/eLife.80622. Elife. 2024. PMID: 39146380 Free PMC article. - CaMKII regulation in information processing and storage.
Coultrap SJ, Bayer KU. Coultrap SJ, et al. Trends Neurosci. 2012 Oct;35(10):607-18. doi: 10.1016/j.tins.2012.05.003. Epub 2012 Jun 19. Trends Neurosci. 2012. PMID: 22717267 Free PMC article. Review.
References
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. - PubMed
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. - PubMed
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources