The adventitia: a progenitor cell niche for the vessel wall - PubMed (original) (raw)
Review
The adventitia: a progenitor cell niche for the vessel wall
Mark W Majesky et al. Cells Tissues Organs. 2012.
Abstract
Recent observations suggest that the adventitial layer of blood vessels exhibits properties resembling a stem/progenitor cell niche. Progenitor cells have been isolated from the adventitia of both murine and human blood vessels with the potential to form endothelial cells, mural cells, osteogenic cells, and adipocytes. These progenitors appear to cluster at or near the border zone between the outer media and inner adventitia. In the mouse, this border zone region corresponds to a localized site of sonic hedgehog signaling in the artery wall. This brief review will discuss the emerging evidence that the tunica adventitia may provide a niche-like signaling environment for resident progenitor cells and will address the role of the adventitia in growth, remodeling, and repair of the artery wall.
Copyright © 2011 S. Karger AG, Basel.
Figures
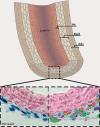
Fig. 1
The adventitia. Schematic view of a large elastic artery in cross section showing organization of the major cell types into layers around a central lumen (top). Int = Intima; Med = media; Adv = adventitia. A segment of the vessel wall (inset) is expanded to show the constituent cell types in the normal, uninjured arterial adventitia (bottom). The left side shows a histological cross section of postnatal day 2 thoracic aorta from a Ptc1-LacZ transgenic mouse stained with nuclear fast red. Note that Shh signaling, as indicated by Ptc1-LacZ activity (blue), is confined to the adventitial layer. The right side shows a false color rendering of the same tissue shown at left depicting CD68+ cells (macrophages, orange), Sca1+ progenitor cells (red), adventitial fibroblasts (green), and T cells (purple). Also present in variable numbers are perivascular nerves and associated glial cells, microvessels of the vasa vasorum network, and adipocytes (see also fig. 2).
Fig. 2
The adventitia as a progenitor cell niche in the artery wall. a The left common carotid artery from a postnatal day 9 mouse was fixed and immunostained for Sca1 expression and examined by confocal microscopy. Shown is a reconstructed low power image of stacked Z-plane confocal images of adventitial Sca1+ progenitor cells. Inset A high power view of the characteristic cluster arrangement of Sca1+ progenitor cells in the mouse carotid artery adventitia. b Schematic representation of a proposed progenitor cell niche in the arterial adventitia. We propose that this niche environment contains soluble factors (orange spheres) including Shh [Passman et al., 2008] and other secreted factors not yet described, and ECM proteins (thin brown, grey and white lines) including various collagens, proteoglycans, and hyaluronic acid. Adventitial progenitor cells will possess matrix adhesion receptors (Y) and cell surface receptors for soluble factors (U) that together signal maintenance of a progenitor phenotype, promote survival of progenitor cells, and prevent their premature differentiation. Upon injury to the artery wall, an influx of inflammatory cells into the adventitia likely directs downregulation of niche signaling leading to differentiation of progenitor cells to form SMCs, pericytes, and possibly other vascular cell types.
Similar articles
- The adventitia: a dynamic interface containing resident progenitor cells.
Majesky MW, Dong XR, Hoglund V, Mahoney WM Jr, Daum G. Majesky MW, et al. Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1530-9. doi: 10.1161/ATVBAHA.110.221549. Arterioscler Thromb Vasc Biol. 2011. PMID: 21677296 Free PMC article. Review. - A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells.
Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. Passman JN, et al. Proc Natl Acad Sci U S A. 2008 Jul 8;105(27):9349-54. doi: 10.1073/pnas.0711382105. Epub 2008 Jun 30. Proc Natl Acad Sci U S A. 2008. PMID: 18591670 Free PMC article. - [Stem and progenitor cells in biostructure of blood vessel walls].
Korta K, Kupczyk P, Skóra J, Pupka A, Zejler P, Hołysz M, Gajda M, Nowakowska B, Barć P, Dorobisz AT, Dawiskiba T, Szyber P, Bar J. Korta K, et al. Postepy Hig Med Dosw (Online). 2013 Sep 18;67:982-95. doi: 10.5604/17322693.1067258. Postepy Hig Med Dosw (Online). 2013. PMID: 24088542 Review. Polish. - Integrated transcriptomics of human blood vessels defines a spatially controlled niche for early mesenchymal progenitor cells.
Wang Y, Thottappillil N, Gomez-Salazar M, Tower RJ, Qin Q, Del Rosario Alvia IC, Xu M, Cherief M, Cheng R, Archer M, Arondekar S, Reddy S, Broderick K, Péault B, James AW. Wang Y, et al. Dev Cell. 2024 Oct 21;59(20):2687-2703.e6. doi: 10.1016/j.devcel.2024.06.015. Epub 2024 Jul 17. Dev Cell. 2024. PMID: 39025061 - Basic Components of Vascular Connective Tissue and Extracellular Matrix.
Halper J. Halper J. Adv Pharmacol. 2018;81:95-127. doi: 10.1016/bs.apha.2017.08.012. Epub 2017 Oct 27. Adv Pharmacol. 2018. PMID: 29310805 Review.
Cited by
- Regenerative Translation of Human Blood-Vessel-Derived MSC Precursors.
Chen WC, Péault B, Huard J. Chen WC, et al. Stem Cells Int. 2015;2015:375187. doi: 10.1155/2015/375187. Epub 2015 Jul 26. Stem Cells Int. 2015. PMID: 26273304 Free PMC article. Review. - Cellular and Molecular Heterogeneity Associated with Vessel Formation Processes.
Castro PR, Barbosa AS, Pereira JM, Ranfley H, Felipetto M, Gonçalves CAX, Paiva IR, Berg BB, Barcelos LS. Castro PR, et al. Biomed Res Int. 2018 Oct 10;2018:6740408. doi: 10.1155/2018/6740408. eCollection 2018. Biomed Res Int. 2018. PMID: 30406137 Free PMC article. Review. - Development and pathologies of the arterial wall.
Seidelmann SB, Lighthouse JK, Greif DM. Seidelmann SB, et al. Cell Mol Life Sci. 2014 Jun;71(11):1977-99. doi: 10.1007/s00018-013-1478-y. Epub 2013 Sep 27. Cell Mol Life Sci. 2014. PMID: 24071897 Free PMC article. Review. - Senescence of endothelial cells promotes phenotypic changes in adventitial fibroblasts: possible implications for vascular aging.
Sarad K, Jankowska U, Skupien-Rabian B, Babler A, Kramann R, Dulak J, Jaźwa-Kusior A. Sarad K, et al. Mol Cell Biochem. 2024 May 14. doi: 10.1007/s11010-024-05028-7. Online ahead of print. Mol Cell Biochem. 2024. PMID: 38743322 - Redistribution of the chromatin remodeler Brg1 directs smooth muscle-derived adventitial progenitor-to-myofibroblast differentiation and vascular fibrosis.
Jolly AJ, Lu S, Dubner AM, Strand KA, Mutryn MF, Pilotti-Riley A, Danis EP, Nemenoff RA, Moulton KS, Majesky MW, Weiser-Evans MC. Jolly AJ, et al. JCI Insight. 2023 May 8;8(9):e164862. doi: 10.1172/jci.insight.164862. JCI Insight. 2023. PMID: 36976650 Free PMC article.
References
- Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. - PubMed
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cell in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
- Brack A.S., Conboy M.J., Roy S., Lee M., Kuo C.J., Keller C., Rando T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL088374/HL/NHLBI NIH HHS/United States
- HL-88374/HL/NHLBI NIH HHS/United States
- HL-93594/HL/NHLBI NIH HHS/United States
- R01 HL093594/HL/NHLBI NIH HHS/United States
- K99 HL087513/HL/NHLBI NIH HHS/United States
- R00 HL087513/HL/NHLBI NIH HHS/United States
- HL-87513/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical