A soluble α-synuclein construct forms a dynamic tetramer - PubMed (original) (raw)
. 2011 Oct 25;108(43):17797-802.
doi: 10.1073/pnas.1113260108. Epub 2011 Oct 17.
Iva Perovic, Johnathan Chittuluru, Alice Kaganovich, Linh T T Nguyen, Jingling Liao, Jared R Auclair, Derrick Johnson, Anuradha Landeru, Alana K Simorellis, Shulin Ju, Mark R Cookson, Francisco J Asturias, Jeffrey N Agar, Brian N Webb, Chulhee Kang, Dagmar Ringe, Gregory A Petsko, Thomas C Pochapsky, Quyen Q Hoang
Affiliations
- PMID: 22006323
- PMCID: PMC3203798
- DOI: 10.1073/pnas.1113260108
A soluble α-synuclein construct forms a dynamic tetramer
Wei Wang et al. Proc Natl Acad Sci U S A. 2011.
Abstract
A heterologously expressed form of the human Parkinson disease-associated protein α-synuclein with a 10-residue N-terminal extension is shown to form a stable tetramer in the absence of lipid bilayers or micelles. Sequential NMR assignments, intramonomer nuclear Overhauser effects, and circular dichroism spectra are consistent with transient formation of α-helices in the first 100 N-terminal residues of the 140-residue α-synuclein sequence. Total phosphorus analysis indicates that phospholipids are not associated with the tetramer as isolated, and chemical cross-linking experiments confirm that the tetramer is the highest-order oligomer present at NMR sample concentrations. Image reconstruction from electron micrographs indicates that a symmetric oligomer is present, with three- or fourfold symmetry. Thermal unfolding experiments indicate that a hydrophobic core is present in the tetramer. A dynamic model for the tetramer structure is proposed, based on expected close association of the amphipathic central helices observed in the previously described micelle-associated "hairpin" structure of α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
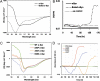
Oligomeric states of αSyn. (A) Elution profile of purified αSyn construct from Superdex75 column. (Inset) Calibration curve used for size estimates. (B) S1 to S4 are molecular weight standards. NP, native purified αSyn; XP, αSyn cross-linked with glutaraldehyde. P1, P2, and P3 are purified cross-linked tetramer, trimer, and monomer, respectively. M17, cross-linked lysate of neuroblastoma cell line M17 overexpressing WT human αSyn. NG, Blue Native PAGE of purified recombinant αSyn (48 refers to the lowest NG band). For analysis of gels, see
SI Appendix, Fig. S1
. (C) MALDI-TOF spectra of αSyn (Top, calculated Mr = 15,397), cross-linked monomer and dimer (Middle, 17 kDa and 35 kDa), and cross-linked trimer and tetramer (Bottom, 52 kDa and 68 kDa).
Fig. 2.
Electron microscopy analysis of purified recombinant αSyn. (A) Image of particles preserved in stain. (Scale bar, 100 nm.) (B) Distribution of particle sizes after glycerol removal. (C) Overall class averages obtained from the small-, medium-, and large-sized particle groups. (Scale bar, 5 nm.) (D and E) Representative class averages from the small- and medium-sized particle groups. (Scale bar, 5 nm.) Symmetry units shown as dashed triangles over the EM class averages.
Fig. 3.
Secondary structure and aggregation of αSyn. (A) Circular dichroism (CD) spectrum of αSyn before (solid line) and after boiling (dotted line). (B) Congo red aggregation assay of αSyn (solid line), boiled αSyn (dashed line), and buffer control with no protein (dotted line). (C) CD spectrum of αSyn wild-type (solid green), mutants A30P (red dashed line), A53T (orange dash), and E46K (blue dash). (D) Congo red aggregation assay of wild-type αSyn (green), A30P (red), E46K (blue), and A53T (orange).
Fig. 4.
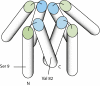
Model for compact αSyn tetramer based on EM reconstruction and PRE. Helices are represented as cylinders. N indicates the N-terminal of the protein, with the first helix (α1, represented by green-ended cylinder) ending at ∼residue 43. The second helix (α2, blue ended) starts ∼residue 50 and ends at residue 103 (marked C). The remainder of the polypeptide, which is expected to be disordered, is not represented. The approximate position of Ser-9 (replaced by Cys for PRE experiments) and Val-82 (maximum PRE on α2) is shown.
Similar articles
- Explaining the structural plasticity of α-synuclein.
Ullman O, Fisher CK, Stultz CM. Ullman O, et al. J Am Chem Soc. 2011 Dec 7;133(48):19536-46. doi: 10.1021/ja208657z. Epub 2011 Nov 14. J Am Chem Soc. 2011. PMID: 22029383 Free PMC article. - The dynamic structure of α-synuclein multimers.
Gurry T, Ullman O, Fisher CK, Perovic I, Pochapsky T, Stultz CM. Gurry T, et al. J Am Chem Soc. 2013 Mar 13;135(10):3865-72. doi: 10.1021/ja310518p. Epub 2013 Feb 27. J Am Chem Soc. 2013. PMID: 23398399 - The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states.
Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, Lansbury PT Jr. Fredenburg RA, et al. Biochemistry. 2007 Jun 19;46(24):7107-18. doi: 10.1021/bi7000246. Epub 2007 May 26. Biochemistry. 2007. PMID: 17530780 - Preparation and Characterization of Stable α-Synuclein Lipoprotein Particles.
Eichmann C, Campioni S, Kowal J, Maslennikov I, Gerez J, Liu X, Verasdonck J, Nespovitaya N, Choe S, Meier BH, Picotti P, Rizo J, Stahlberg H, Riek R. Eichmann C, et al. J Biol Chem. 2016 Apr 15;291(16):8516-27. doi: 10.1074/jbc.M115.707968. Epub 2016 Feb 4. J Biol Chem. 2016. PMID: 26846854 Free PMC article. - Bacterial in-cell NMR of human α-synuclein: a disordered monomer by nature?
Binolfi A, Theillet FX, Selenko P. Binolfi A, et al. Biochem Soc Trans. 2012 Oct;40(5):950-4. doi: 10.1042/BST20120096. Biochem Soc Trans. 2012. PMID: 22988846 Review.
Cited by
- Identification and quantification of within-burst dynamics in singly labeled single-molecule fluorescence lifetime experiments.
Harris PD, Lerner E. Harris PD, et al. Biophys Rep (N Y). 2022 Sep 14;2(3):100071. doi: 10.1016/j.bpr.2022.100071. Epub 2022 Aug 17. Biophys Rep (N Y). 2022. PMID: 36204594 Free PMC article. - ATP13A2 and Alpha-synuclein: a Metal Taste in Autophagy.
Lopes da Fonseca T, Outeiro TF. Lopes da Fonseca T, et al. Exp Neurobiol. 2014 Dec;23(4):314-23. doi: 10.5607/en.2014.23.4.314. Epub 2014 Dec 12. Exp Neurobiol. 2014. PMID: 25548531 Free PMC article. Review. - Synthetic Proteins and Peptides for the Direct Interrogation of α-Synuclein Posttranslational Modifications.
Pratt MR, Abeywardana T, Marotta NP. Pratt MR, et al. Biomolecules. 2015 Jun 25;5(3):1210-27. doi: 10.3390/biom5031210. Biomolecules. 2015. PMID: 26120904 Free PMC article. Review. - Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences.
Exner N, Lutz AK, Haass C, Winklhofer KF. Exner N, et al. EMBO J. 2012 Jun 26;31(14):3038-62. doi: 10.1038/emboj.2012.170. EMBO J. 2012. PMID: 22735187 Free PMC article. Review. - Investigation of the Polymeric Properties of α-Synuclein and Comparison with NMR Experiments: A Replica Exchange Molecular Dynamics Study.
Narayanan C, Weinstock DS, Wu KP, Baum J, Levy RM. Narayanan C, et al. J Chem Theory Comput. 2012 Oct 9;8(10):3929-3942. doi: 10.1021/ct300241t. Epub 2012 Jun 5. J Chem Theory Comput. 2012. PMID: 23162382 Free PMC article.
References
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. - PubMed
- Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):S2–S12. - PubMed
- Klockgether T. Parkinson's disease: Clinical aspects. Cell Tissue Res. 2004;318(1):115–120. - PubMed
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials