Protein release through nonlethal oncotic pores as an alternative nonclassical secretory pathway - PubMed (original) (raw)
Protein release through nonlethal oncotic pores as an alternative nonclassical secretory pathway
William J Chirico. BMC Cell Biol. 2011.
Abstract
Background: Nonclassical (unconventional) protein secretion is thought to represent the primary secretion mechanism for several cytosolic proteins, such as HIV-Tat, galectin 1, interleukin-1β, and several proteins that shuttle between the nucleus and cytosol, such as fibroblast growth factor 1 (FGF1), FGF2, and nucleolin. Four nonclassical secretory pathways have been described including direct transport (presumably through transporters in the plasma membrane), secretion via exosomes, lysosomal secretion, and blebbing. The purpose of this study was to gain mechanistic insight into nonclassical protein secretion using phosphoglycerate kinase 1 (PGK1), a previously identified nonclassical secretory protein, as a reporter protein.
Results: Upon shifting HeLa cells into serum-free media PGK1 was released as a free soluble protein without cell loss. Release occurred in two phases: a rapid early phase and a slow late phase. Using a repertory of inhibitors, PGK1 release was shown not to rely on the classical secretory pathway. However, components of the cytoskeleton partially contributed to its release. Significantly, the presence of serum or bovine serum albumin in the media inhibited PGK1 release.
Conclusions: These results are consistent with a novel model of protein release termed oncotic release, in which a change in the colloidal osmotic pressure (oncotic pressure) upon serum withdrawal creates nonlethal oncotic pores in the plasma membrane through which PGK1 - and likely other nearby proteins - are released before the pores are rapidly resealed. These findings identify an alternative mechanism of release for FGF1, HIV-Tat, and galectin 1 whose reported nonclassical secretion is induced by serum withdrawal. Oncotic release may occur in routine cell biological experiments during which cells are washed with serum-free buffers or media and in pathophysiological conditions, such as edema, during which extracellular protein concentrations change.
Figures
Figure 1
Effect of cell density on PGK1 release. HeLa cells were plated at the indicated cell densities in MEM/10% NBS. After 24 h, the cells were washed with serum-free MEM and then incubated with the same media for 2 h. The amount of PGK1 released into the media was determined using an ELISA. Error bars represent the s.d. of the mean (n = 4). AU, arbitrary units.
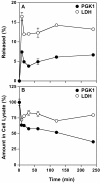
Figure 2
Time course of PGK1 release. After HeLa cells were grown in MEM/10% NBS for 24 h, the cells were washed once with serum-free MEM and then incubated in the same media for the indicated times. The amount of PGK1 and LDH released (A) and remaining in cell lysates (B) was determined and expressed as a percent of the total of each protein in cell lysates at 0 min (untreated cells). Error bars represent the s.d. of the mean (n = 4).
Figure 3
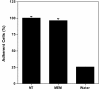
Cell loss does not accompany serum-withdrawal. HeLa cells were grown for 24 h in MEM/10% NCS and then either left untreated (NT) or washed once with serum-free MEM and then incubated in the same media for 1 h (MEM). A parallel set of samples was treated with water (Water). Cell number was assayed using SYTOX Green and expressed as the percent remaining relative to the untreated sample. Error bars represent the s.d. of the mean (n = 4).
Figure 4
PGK1 released as a free soluble protein. Media was collected from HeLa cell cultures 7.5 min after serum withdrawal and then treated with (lanes 2 and 4) or without Proteinase K (PK, lanes 1 and 3) in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of Triton X-100 (Triton) as described in Materials and Methods. Samples were separated on SDS-PAGE gels and then immunoblotted with anti-PGK1 antibodies.
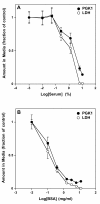
Figure 5
Serum or BSA blocks release of PGK1 and LDH. HeLa cells were grown for 24 h in MEM containing 10% serum. After 24 h, media was removed, the cells washed with MEM containing the indicated amounts of serum (A) or BSA (B), and then incubated with the same media for 7.5 min. The media was collected and the amount of PGK1 and LDH was quantified as described in Materials and Methods. Values are expressed as the ratio of the amount of each protein in the media at each concentration of serum or BSA relative to that in the absence of serum or BSA, respectively. For graphing purposes, the sample lacking serum was defined as 0.001% serum and that lacking BSA as 0.01 mg/ml BSA. Error bars represent the s.d. of the mean (n = 4).
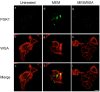
Figure 6
Confocal images of PGK1 at the cell surface. HeLa cells were grown in MEM supplemented with 10% NBS. After 24 h, they were either left untreated (a-c), washed once with serum-free MEM and then incubated with the same media for 30 min (d-f), or washed once with serum-free MEM containing BSA and then incubated with the same media for 30 min (g-i). After the incubations the cells were fixed as described in Materials and Methods. Cells were not permeabilized with detergents in order to detect only cell surface proteins. The cell surface was detected using wheat germ agglutinin (WGA, red) and PGK1 (green) was localized using affinity purified rabbit anti-human PGK1 antibody. The bar represents 10 μm (i).
Similar articles
- A sandwich ELISA for phosphoglycerate kinase.
Zhao W, Pao S, Malik F, Soh J, Fernandez S, Chirico WJ. Zhao W, et al. J Immunoassay Immunochem. 2008;29(3):220-33. doi: 10.1080/15321810802119588. J Immunoassay Immunochem. 2008. PMID: 18569371 - AHNAK2 Participates in the Stress-Induced Nonclassical FGF1 Secretion Pathway.
Kirov A, Kacer D, Conley BA, Vary CP, Prudovsky I. Kirov A, et al. J Cell Biochem. 2015 Aug;116(8):1522-31. doi: 10.1002/jcb.25047. J Cell Biochem. 2015. PMID: 25560297 Free PMC article. - Oncotic pressure and edema formation in hypoalbuminemic HIV-infected patients with proteinuria.
Guardia JA, Ortiz-Butcher C, Bourgoignie JJ. Guardia JA, et al. Am J Kidney Dis. 1997 Dec;30(6):822-8. doi: 10.1016/s0272-6386(97)90088-3. Am J Kidney Dis. 1997. PMID: 9398127 - A Role for Liquid-Ordered Plasma Membrane Nanodomains Coordinating the Unconventional Secretory Pathway of Fibroblast Growth Factor 2?
Lolicato F, Nickel W. Lolicato F, et al. Front Cell Dev Biol. 2022 Apr 1;10:864257. doi: 10.3389/fcell.2022.864257. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35433697 Free PMC article. Review. - [Nonclassical mechanisms of secretory protein in eukaryotic cells].
Zhang NN, Liu X, Sun J, Wu Y, Li QW. Zhang NN, et al. Yi Chuan. 2009 Jan;31(1):29-35. doi: 10.3724/sp.j.1005.2009.00029. Yi Chuan. 2009. PMID: 19138898 Review. Chinese.
Cited by
- Galectin-3: a possible complementary marker to the PSA blood test.
Balan V, Wang Y, Nangia-Makker P, Kho D, Bajaj M, Smith D, Heilbrun L, Raz A, Heath E. Balan V, et al. Oncotarget. 2013 Apr;4(4):542-9. doi: 10.18632/oncotarget.923. Oncotarget. 2013. PMID: 23625538 Free PMC article. - More complete polarization of renal tubular epithelial cells by artificial urine.
Vinaiphat A, Charngkaew K, Thongboonkerd V. Vinaiphat A, et al. Cell Death Discov. 2018 Oct 10;4:47. doi: 10.1038/s41420-018-0112-z. eCollection 2018. Cell Death Discov. 2018. PMID: 30323952 Free PMC article. - Plasma and urinary heme oxygenase-1 in AKI.
Zager RA, Johnson AC, Becker K. Zager RA, et al. J Am Soc Nephrol. 2012 Jun;23(6):1048-57. doi: 10.1681/ASN.2011121147. Epub 2012 Mar 22. J Am Soc Nephrol. 2012. PMID: 22440905 Free PMC article. - Through the back door: Unconventional protein secretion.
Cohen MJ, Chirico WJ, Lipke PN. Cohen MJ, et al. Cell Surf. 2020 Sep 15;6:100045. doi: 10.1016/j.tcsw.2020.100045. eCollection 2020 Dec. Cell Surf. 2020. PMID: 33225116 Free PMC article. Review. - Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer.
Angelova AL, Grekova SP, Heller A, Kuhlmann O, Soyka E, Giese T, Aprahamian M, Bour G, Rüffer S, Cziepluch C, Daeffler L, Rommelaere J, Werner J, Raykov Z, Giese NA. Angelova AL, et al. J Virol. 2014 May;88(10):5263-76. doi: 10.1128/JVI.03688-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574398 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous