An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28 - PubMed (original) (raw)
doi: 10.1038/jcbfm.2011.147. Epub 2011 Oct 19.
Astrid J Yeo, Roger N Gunn, Kijoung Song, Graham Wadsworth, Andrew Lewis, Chris Rhodes, David J Pulford, Idriss Bennacef, Christine A Parker, Pamela L StJean, Lon R Cardon, Vincent E Mooser, Paul M Matthews, Eugenii A Rabiner, Justin P Rubio
Affiliations
- PMID: 22008728
- PMCID: PMC3323305
- DOI: 10.1038/jcbfm.2011.147
An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28
David R Owen et al. J Cereb Blood Flow Metab. 2012 Jan.
Abstract
[(11)C]PBR28 binds the 18-kDa Translocator Protein (TSPO) and is used in positron emission tomography (PET) to detect microglial activation. However, quantitative interpretations of signal are confounded by large interindividual variability in binding affinity, which displays a trimodal distribution compatible with a codominant genetic trait. Here, we tested directly for an underlying genetic mechanism to explain this. Binding affinity of PBR28 was measured in platelets isolated from 41 human subjects and tested for association with polymorphisms in TSPO and genes encoding other proteins in the TSPO complex. Complete agreement was observed between the TSPO Ala147Thr genotype and PBR28 binding affinity phenotype (P value=3.1 × 10(-13)). The TSPO Ala147Thr polymorphism predicts PBR28 binding affinity in human platelets. As all second-generation TSPO PET radioligands tested hitherto display a trimodal distribution in binding affinity analogous to PBR28, testing for this polymorphism may allow quantitative interpretation of TSPO PET studies with these radioligands.
Figures
Figure 1
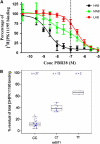
(A) Competition binding assay using unlabelled PBR28 to displace [3H]PK11195 in human platelets isolated from whole blood (_n_=41). The dashed vertical line indicates the concentration of PBR28 used to generate panel B. The fractional binding is described by the following equations, One site model
Two site model
where B, binding signal; NS, nonspecific binding; K i, binding affinity; and _f_H, the fraction of high-affinity binding sites. (B) Box-whisker plot of the residual [3H]PK11195 binding in the presence of 100 nmol/L unlabelled PBR28 (expressed as a percentage of the total [3H]PK11195 binding in the absence of PBR28) stratified on rs6971 genotype. Percentage residual of total binding is plotted as blue diamonds for each individual. HAB, high affinity binder; LAB, low affinity binder; MAB, mixed affinity binder.
Similar articles
- A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation.
Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB; Biomarkers Consortium PET Radioligand Project Team. Kreisl WC, et al. J Cereb Blood Flow Metab. 2013 Jan;33(1):53-8. doi: 10.1038/jcbfm.2012.131. Epub 2012 Sep 12. J Cereb Blood Flow Metab. 2013. PMID: 22968319 Free PMC article. - Mixed-affinity binding in humans with 18-kDa translocator protein ligands.
Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, Innis RB, Pike VW, Reynolds R, Matthews PM, Parker CA. Owen DR, et al. J Nucl Med. 2011 Jan;52(1):24-32. doi: 10.2967/jnumed.110.079459. Epub 2010 Dec 13. J Nucl Med. 2011. PMID: 21149489 Free PMC article. - Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA.
Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I, De Luca V, Wilson AA, Houle S. Mizrahi R, et al. J Cereb Blood Flow Metab. 2012 Jun;32(6):968-72. doi: 10.1038/jcbfm.2012.46. Epub 2012 Apr 4. J Cereb Blood Flow Metab. 2012. PMID: 22472607 Free PMC article. Clinical Trial. - Have (R)-[11C]PK11195 challengers fulfilled the promise? A scoping review of clinical TSPO PET studies.
Chauveau F, Becker G, Boutin H. Chauveau F, et al. Eur J Nucl Med Mol Imaging. 2021 Dec;49(1):201-220. doi: 10.1007/s00259-021-05425-w. Epub 2021 Aug 13. Eur J Nucl Med Mol Imaging. 2021. PMID: 34387719 Free PMC article. Review. - Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands.
Owen DR, Matthews PM. Owen DR, et al. Int Rev Neurobiol. 2011;101:19-39. doi: 10.1016/B978-0-12-387718-5.00002-X. Int Rev Neurobiol. 2011. PMID: 22050847 Review.
Cited by
- Roles of microglia in brain development, tissue maintenance and repair.
Michell-Robinson MA, Touil H, Healy LM, Owen DR, Durafourt BA, Bar-Or A, Antel JP, Moore CS. Michell-Robinson MA, et al. Brain. 2015 May;138(Pt 5):1138-59. doi: 10.1093/brain/awv066. Epub 2015 Mar 29. Brain. 2015. PMID: 25823474 Free PMC article. Review. - The development status of PET radiotracers for evaluating neuroinflammation.
Lee N, Choi JY, Ryu YH. Lee N, et al. Nucl Med Mol Imaging. 2024 Jun;58(4):160-176. doi: 10.1007/s13139-023-00831-4. Epub 2024 Jan 8. Nucl Med Mol Imaging. 2024. PMID: 38932754 Free PMC article. Review. - PET imaging of brain inflammation during early epileptogenesis in a rat model of temporal lobe epilepsy.
Dedeurwaerdere S, Callaghan PD, Pham T, Rahardjo GL, Amhaoul H, Berghofer P, Quinlivan M, Mattner F, Loc'h C, Katsifis A, Grégoire MC. Dedeurwaerdere S, et al. EJNMMI Res. 2012 Nov 8;2(1):60. doi: 10.1186/2191-219X-2-60. EJNMMI Res. 2012. PMID: 23136853 Free PMC article. - Comparative Evaluation of Three TSPO PET Radiotracers in a LPS-Induced Model of Mild Neuroinflammation in Rats.
Sridharan S, Lepelletier FX, Trigg W, Banister S, Reekie T, Kassiou M, Gerhard A, Hinz R, Boutin H. Sridharan S, et al. Mol Imaging Biol. 2017 Feb;19(1):77-89. doi: 10.1007/s11307-016-0984-3. Mol Imaging Biol. 2017. PMID: 27481358 Free PMC article. - Ibudilast (MN-166) in amyotrophic lateral sclerosis- an open label, safety and pharmacodynamic trial.
Babu S, Hightower BG, Chan J, Zürcher NR, Kivisäkk P, Tseng CJ, Sanders DL, Robichaud A, Banno H, Evora A, Ashokkumar A, Pothier L, Paganoni S, Chew S, Dojillo J, Matsuda K, Gudesblatt M, Berry JD, Cudkowicz ME, Hooker JM, Atassi N. Babu S, et al. Neuroimage Clin. 2021;30:102672. doi: 10.1016/j.nicl.2021.102672. Epub 2021 Apr 15. Neuroimage Clin. 2021. PMID: 34016561 Free PMC article.
References
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123 (Pt 11:2321–2337. - PubMed
- Chauveau F, Boutin H, Van CN, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–2319. - PubMed
- Costa B, Pini S, Gabelloni P, Da PE, Abelli M, Lari L, Preve M, Lucacchini A, Cassano GB, Martini C. The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology. 2009a;150:5438–5445. - PubMed
- Costa B, Pini S, Martini C, Abelli M, Gabelloni P, Landi S, Muti M, Gesi C, Lari L, Cardini A, Galderisi S, Mucci A, Lucacchini A, Cassano GB. Ala147Thr substitution in translocator protein is associated with adult separation anxiety in patients with depression. Psychiatr Genet. 2009b;19:110–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials